A systematic review of the effectiveness of adalimumab
A systematic review of the effectiveness of adalimumab
A systematic review of the effectiveness of adalimumab
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
66<br />
Effectiveness<br />
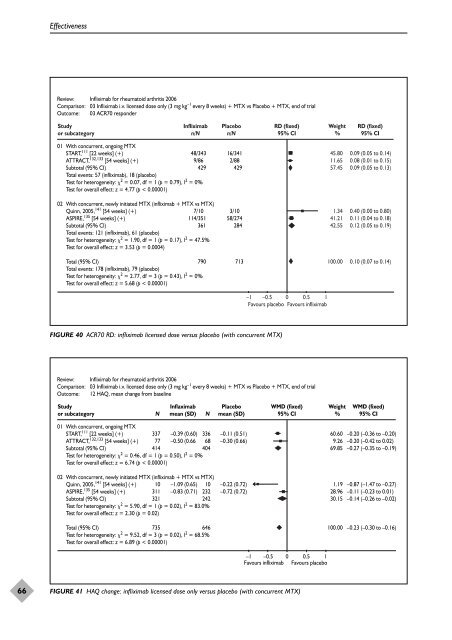
Review: Infliximab for rheumatoid arthritis 2006<br />
Comparison: 03 Infliximab i.v. licensed dose only (3 mg kg –1 every 8 weeks) + MTX vs Placebo + MTX, end <strong>of</strong> trial<br />
Outcome: 03 ACR70 responder<br />
Study<br />
or subcategory<br />
Infliximab<br />
n/N<br />
Placebo<br />
n/N<br />
01 With concurrent, ongoing MTX<br />
START, 111 [22 weeks] (+)<br />
ATTRACT, 132,133 [54 weeks] (+)<br />
Subtotal (95% CI)<br />
Total events: 57 (infliximab), 18 (placebo)<br />
Test for heterogeneity: 2 = 0.07, df = 1 (p = 0.79), I2 48/343 16/341<br />
9/86 2/88<br />
429 429<br />
= 0%<br />
Test for overall effect: z = 4.77 (p < 0.00001)<br />
02 With concurrent, newly initiated MTX (infliximab + MTX vs MTX)<br />
Quinn, 2005, 141 [54 weeks] (+)<br />
ASPIRE, 135 [54 weeks] (+)<br />
Subtotal (95% CI)<br />
Total events: 121 (infliximab), 61 (placebo)<br />
Test for heterogeneity: 2 = 1.90, df = 1 (p = 0.17), I2 7/10 3/10<br />
114/351 58/274<br />
361 284<br />
= 47.5%<br />
Test for overall effect: z = 3.53 (p = 0.0004)<br />
Total (95% CI)<br />
Total events: 178 (infliximab), 79 (placebo)<br />
Test for heterogeneity: 2 = 2.77, df = 3 (p = 0.43), I2 790 713<br />
= 0%<br />
Test for overall effect: z = 5.68 (p < 0.00001)<br />
FIGURE 40 ACR70 RD: infliximab licensed dose versus placebo (with concurrent MTX)<br />
RD (fixed)<br />
95% CI<br />
–1 –0.5 0 0.5 1<br />
Favours placebo Favours infliximab<br />
Review: Infliximab for rheumatoid arthritis 2006<br />
Comparison: 03 Infliximab i.v. licensed dose only (3 mg kg –1 every 8 weeks) + MTX vs Placebo + MTX, end <strong>of</strong> trial<br />
Outcome: 12 HAQ, mean change from baseline<br />
Study<br />
or subcategory<br />
01 With concurrent, ongoing MTX<br />
START, 111 [22 weeks] (+)<br />
ATTRACT, 132,133 [54 weeks] (+)<br />
Subtotal (95% CI)<br />
N<br />
Inflaximab<br />
mean (SD) N<br />
Test for heterogeneity: 2 = 0.46, df = 1 (p = 0.50), I 2 = 0%<br />
Test for overall effect: z = 6.74 (p < 0.00001)<br />
02 With concurrent, newly initiated MTX (infliximab + MTX vs MTX)<br />
Quinn, 2005, 141 [54 weeks] (+)<br />
ASPIRE, 135 [54 weeks] (+)<br />
Subtotal (95% CI)<br />
Placebo<br />
mean (SD)<br />
337 –0.39 (0.60) 336 –0.11 (0.51)<br />
77 –0.50 (0.66 68 –0.30 (0.66)<br />
414 404<br />
10 –1.09 (0.65) 10 –0.22 (0.72)<br />
311 –0.83 (0.71) 232 –0.72 (0.72)<br />
321 242<br />
Test for heterogeneity: 2 = 5.90, df = 1 (p = 0.02), I 2 = 83.0%<br />
Test for overall effect: z = 2.30 (p = 0.02)<br />
Total (95% CI)<br />
Test for heterogeneity: 2 = 9.52, df = 3 (p = 0.02), I2 735 646<br />
= 68.5%<br />
Test for overall effect: z = 6.89 (p < 0.00001)<br />
WMD (fixed)<br />
95% CI<br />
–1 –0.5 0 0.5 1<br />
Favours infliximab Favours placebo<br />
FIGURE 41 HAQ change: infliximab licensed dose only versus placebo (with concurrent MTX)<br />
Weight<br />
%<br />
45.80<br />
11.65<br />
57.45<br />
1.34<br />
41.21<br />
42.55<br />
100.00<br />
Weight<br />
%<br />
60.60<br />
9.26<br />
69.85<br />
1.19<br />
28.96<br />
30.15<br />
100.00<br />
RD (fixed)<br />
95% CI<br />
0.09 (0.05 to 0.14)<br />
0.08 (0.01 to 0.15)<br />
0.09 (0.05 to 0.13)<br />
0.40 (0.00 to 0.80)<br />
0.11 (0.04 to 0.18)<br />
0.12 (0.05 to 0.19)<br />
0.10 (0.07 to 0.14)<br />
WMD (fixed)<br />
95% CI<br />
–0.20 (–0.36 to –0.20)<br />
–0.20 (–0.42 to 0.02)<br />
–0.27 (–0.35 to –0.19)<br />
–0.87 (–1.47 to –0.27)<br />
–0.11 (–0.23 to 0.01)<br />
–0.14 (–0.26 to –0.02)<br />
–0.23 (–0.30 to –0.16)