A systematic review of the effectiveness of adalimumab
A systematic review of the effectiveness of adalimumab
A systematic review of the effectiveness of adalimumab
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
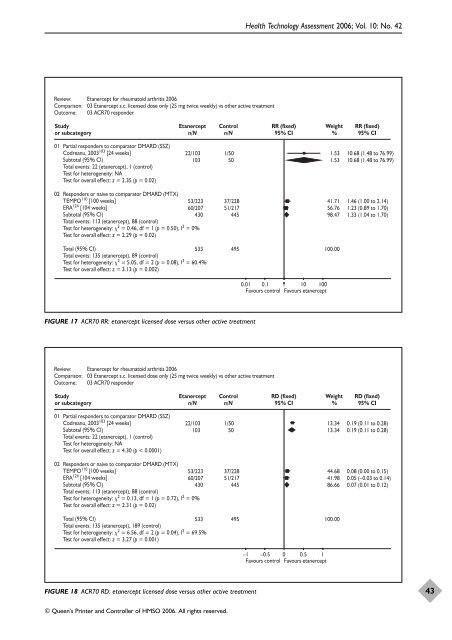
Review: Etanercept for rheumatoid arthritis 2006<br />
Comparison: 03 Etanercept s.c. licensed dose only (25 mg twice weekly) vs o<strong>the</strong>r active treatment<br />
Outcome: 03 ACR70 responder<br />
Study<br />
or subcategory<br />
01 Partial responders to comparator DMARD (SSZ)<br />
Codreanu, 2003 103 [24 weeks]<br />
Subtotal (95% CI)<br />
Total events: 22 (etanercept), 1 (control)<br />
Test for heterogeneity: NA<br />
Test for overall effect: z = 2.35 (p = 0.02)<br />
Etanercept<br />
n/N<br />
© Queen’s Printer and Controller <strong>of</strong> HMSO 2006. All rights reserved.<br />
Control<br />
n/N<br />
22/103 1/50<br />
103 50<br />
02 Responders or naive to comparator DMARD (MTX)<br />
TEMPO110 [100 weeks]<br />
ERA124 [104 weeks]<br />
Subtotal (95% CI)<br />
Total events: 113 (etanercept), 88 (control)<br />
Test for heterogeneity: 2 = 0.46, df = 1 (p = 0.50), I2 53/223<br />
60/207<br />
37/228<br />
51/217<br />
430 445<br />
= 0%<br />
Test for overall effect: z = 2.29 (p = 0.02)<br />
Total (95% CI)<br />
Total events: 135 (etanercept), 89 (control)<br />
Test for heterogeneity: 2 = 5.05, df = 2 (p = 0.08), I2 533 495<br />
= 60.4%<br />
Test for overall effect: z = 3.13 (p = 0.002)<br />
FIGURE 17 ACR70 RR: etanercept licensed dose versus o<strong>the</strong>r active treatment<br />
Review: Etanercept for rheumatoid arthritis 2006<br />
Comparison: 03 Etanercept s.c. licensed dose only (25 mg twice weekly) vs o<strong>the</strong>r active treatment<br />
Outcome: 03 ACR70 responder<br />
Study<br />
or subcategory<br />
01 Partial responders to comparator DMARD (SSZ)<br />
Codreanu, 2003 103 [24 weeks]<br />
Subtotal (95% CI)<br />
Total events: 22 (etanercept), 1 (control)<br />
Test for heterogeneity: NA<br />
Test for overall effect: z = 4.30 (p < 0.0001)<br />
Etanercept<br />
n/N<br />
Control<br />
n/N<br />
22/103 1/50<br />
103 50<br />
02 Responders or naive to comparator DMARD (MTX)<br />
TEMPO110 [100 weeks]<br />
ERA124 [104 weeks]<br />
Subtotal (95% CI)<br />
Total events: 113 (etanercept), 88 (control)<br />
Test for heterogeneity: 2 = 0.13, df = 1 (p = 0.72), I2 53/223<br />
60/207<br />
37/228<br />
51/217<br />
430 445<br />
= 0%<br />
Test for overall effect: z = 2.31 (p = 0.02)<br />
Total (95% CI)<br />
Total events: 135 (etanercept), 189 (control)<br />
Test for heterogeneity: 2 = 6.56, df = 2 (p = 0.04), I2 533 495<br />
= 69.5%<br />
Test for overall effect: z = 3.27 (p = 0.001)<br />
FIGURE 18 ACR70 RD: etanercept licensed dose versus o<strong>the</strong>r active treatment<br />
Health Technology Assessment 2006; Vol. 10: No. 42<br />
RR (fixed)<br />
95% CI<br />
0.01 0.1 1 10 100<br />
Favours control Favours etanercept<br />
RD (fixed)<br />
95% CI<br />
–1 –0.5 0 0.5 1<br />
Favours control Favours etanercept<br />
Weight<br />
%<br />
1.53<br />
1.53<br />
41.71<br />
56.76<br />
98.47<br />
100.00<br />
Weight<br />
%<br />
13.34<br />
13.34<br />
44.68<br />
41.98<br />
86.66<br />
100.00<br />
RR (fixed)<br />
95% CI<br />
10.68 (1.48 to 76.99)<br />
10.68 (1.48 to 76.99)<br />
1.46 (1.00 to 2.14)<br />
1.23 (0.89 to 1.70)<br />
1.33 (1.04 to 1.70)<br />
RD (fixed)<br />
95% CI<br />
0.19 (0.11 to 0.28)<br />
0.19 (0.11 to 0.28)<br />
0.08 (0.00 to 0.15)<br />
0.05 (–0.03 to 0.14)<br />
0.07 (0.01 to 0.12)<br />
43