A systematic review of the effectiveness of adalimumab
A systematic review of the effectiveness of adalimumab
A systematic review of the effectiveness of adalimumab
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
management <strong>of</strong> RA, i.e. 1st and 2nd line”.<br />
Etanercept and methotrexate were used in<br />
combination as “<strong>the</strong> body <strong>of</strong> evidence suggests<br />
that combination <strong>the</strong>rapy is more effective than<br />
mono<strong>the</strong>rapy”. Using combination data, however,<br />
will weigh ICERs in favour <strong>of</strong> etanercept since<br />
patients responding to combined <strong>the</strong>rapy, if <strong>the</strong>y<br />
are DMARD naïve, have <strong>the</strong> opportunity <strong>of</strong><br />
responding to two agents and many may have<br />
responded to methotrexate alone.<br />
The model uses 6-monthly cycles and allows<br />
patients to: experience changes in disease severity;<br />
enter a remission state; develop drug tolerance<br />
problems; experience an SAE; or die. At <strong>the</strong> end<br />
<strong>of</strong> each 6-month cycle <strong>the</strong> patient can:<br />
● change disease severity<br />
● experience an SAE<br />
● switch treatment <strong>the</strong>rapy<br />
● die.<br />
The model run consisted <strong>of</strong> 10,000 hypo<strong>the</strong>tical<br />
patients, followed until death. Costs were<br />
calculated from <strong>the</strong> perspective <strong>of</strong> a healthcare<br />
provider. The main driver <strong>of</strong> <strong>the</strong> model result is<br />
<strong>the</strong> patient’s disease severity. Disease severity<br />
determines several factors in <strong>the</strong> model, including<br />
<strong>the</strong> likelihood <strong>of</strong> switching <strong>the</strong>rapy, health-related<br />
utility and mortality. HAQ was used to represent<br />
disease severity as it was not practical to measure<br />
both HAQ and DAS28 scores simultaneously.<br />
However, for <strong>the</strong> purpose <strong>of</strong> ‘switching thresholds’<br />
a relationship between HAQ and DAS28 was<br />
required and changes in HAQ score were used as a<br />
proxy for changes in <strong>the</strong> DAS28. Perhaps here it<br />
would have been more appropriate to use actual<br />
switching rates from clinical observation ra<strong>the</strong>r<br />
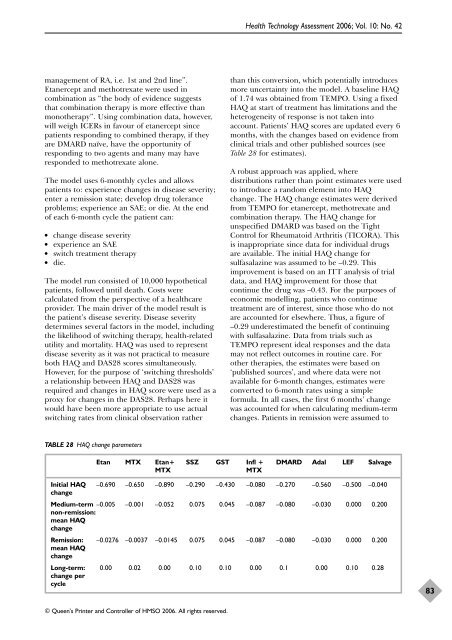
TABLE 28 HAQ change parameters<br />
© Queen’s Printer and Controller <strong>of</strong> HMSO 2006. All rights reserved.<br />
Health Technology Assessment 2006; Vol. 10: No. 42<br />
than this conversion, which potentially introduces<br />
more uncertainty into <strong>the</strong> model. A baseline HAQ<br />
<strong>of</strong> 1.74 was obtained from TEMPO. Using a fixed<br />
HAQ at start <strong>of</strong> treatment has limitations and <strong>the</strong><br />
heterogeneity <strong>of</strong> response is not taken into<br />
account. Patients’ HAQ scores are updated every 6<br />
months, with <strong>the</strong> changes based on evidence from<br />
clinical trials and o<strong>the</strong>r published sources (see<br />
Table 28 for estimates).<br />
A robust approach was applied, where<br />
distributions ra<strong>the</strong>r than point estimates were used<br />
to introduce a random element into HAQ<br />
change. The HAQ change estimates were derived<br />
from TEMPO for etanercept, methotrexate and<br />
combination <strong>the</strong>rapy. The HAQ change for<br />
unspecified DMARD was based on <strong>the</strong> Tight<br />
Control for Rheumatoid Arthritis (TICORA). This<br />
is inappropriate since data for individual drugs<br />
are available. The initial HAQ change for<br />
sulfasalazine was assumed to be –0.29. This<br />
improvement is based on an ITT analysis <strong>of</strong> trial<br />
data, and HAQ improvement for those that<br />
continue <strong>the</strong> drug was –0.43. For <strong>the</strong> purposes <strong>of</strong><br />
economic modelling, patients who continue<br />
treatment are <strong>of</strong> interest, since those who do not<br />
are accounted for elsewhere. Thus, a figure <strong>of</strong><br />
–0.29 underestimated <strong>the</strong> benefit <strong>of</strong> continuing<br />
with sulfasalazine. Data from trials such as<br />
TEMPO represent ideal responses and <strong>the</strong> data<br />
may not reflect outcomes in routine care. For<br />
o<strong>the</strong>r <strong>the</strong>rapies, <strong>the</strong> estimates were based on<br />
‘published sources’, and where data were not<br />
available for 6-month changes, estimates were<br />
converted to 6-month rates using a simple<br />
formula. In all cases, <strong>the</strong> first 6 months’ change<br />
was accounted for when calculating medium-term<br />
changes. Patients in remission were assumed to<br />
Etan MTX Etan+ SSZ GST Infl + DMARD Adal LEF Salvage<br />
MTX MTX<br />
Initial HAQ<br />
change<br />
–0.690 –0.650 –0.890 –0.290 –0.430 –0.080 –0.270 –0.560 –0.500 –0.040<br />
Medium-term –0.005<br />
non-remission:<br />
mean HAQ<br />
change<br />
–0.001 –0.052 0.075 0.045 –0.087 –0.080 –0.030 0.000 0.200<br />
Remission:<br />
mean HAQ<br />
change<br />
–0.0276 –0.0037 –0.0145 0.075 0.045 –0.087 –0.080 –0.030 0.000 0.200<br />
Long-term: 0.00 0.02 0.00 0.10 0.10 0.00 0.1 0.00 0.10 0.28<br />
change per<br />
cycle<br />
83