A systematic review of the effectiveness of adalimumab
A systematic review of the effectiveness of adalimumab
A systematic review of the effectiveness of adalimumab
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
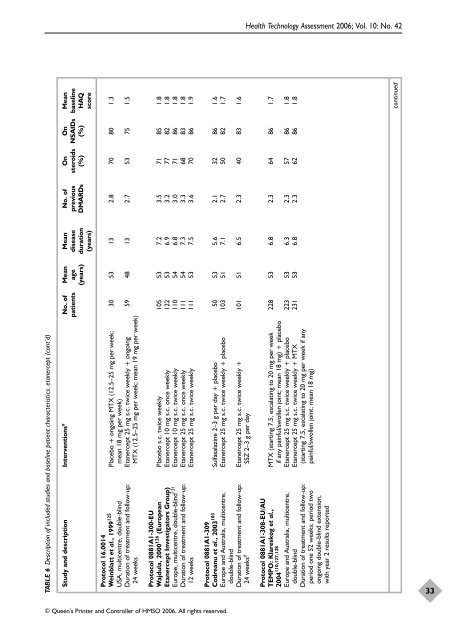
TABLE 6 Description <strong>of</strong> included studies and baseline patient characteristics: etanercept (cont’d)<br />
No. <strong>of</strong> Mean Mean No. <strong>of</strong> On On Mean<br />
patients age disease previous steroids NSAIDs baseline<br />
(years) duration DMARDs (%) (%) HAQ<br />
(years) score<br />
Study and description Interventions a<br />
Protocol 16.0014<br />
Weinblatt et al., 1999 125<br />
Placebo + ongoing MTX (12.5–25 mg per week; 30 53 13 2.8 70 80 1.3<br />
USA, multicentre, double-blind mean 18 mg per week)<br />
Duration <strong>of</strong> treatment and follow-up: Etanercept 25 mg s.c. twice weekly + ongoing 59 48 13 2.7 53 75 1.5<br />
24 weeks MTX (12.5–25 mg per week; mean 19 mg per week)<br />
Protocol 0881A1-300-EU<br />
Wajdula, 2000 126 (European Placebo s.c. twice weekly 105 53 7.2 3.5 71 85 1.8<br />
Etanercept Investigators Group) Etanercept 10 mg s.c. once weekly 122 53 6.9 3.2 77 82 1.8<br />
Europe, multicentre, double-blind 131<br />
Etanercept 10 mg s.c. twice weekly 110 54 6.8 3.0 71 86 1.8<br />
Duration <strong>of</strong> treatment and follow-up: Etanercept 25 mg s.c. once weekly 111 54 7.3 3.3 68 83 1.8<br />
12 weeks Etanercept 25 mg s.c. twice weekly 111 53 7.5 3.6 70 86 1.9<br />
Protocol 0881A1-309<br />
Codreanu et al., 2003 103<br />
Sulfasalazine 2–3 g per day + placebo 50 53 5.6 2.1 32 86 1.6<br />
Europe and Australia, multicentre, Etanercept 25 mg s.c. twice weekly + placebo 103 51 7.1 2.7 50 82 1.7<br />
double-blind<br />
Duration <strong>of</strong> treatment and follow-up: Etanercept 25 mg s.c. twice weekly + 101 51 6.5 2.3 40 83 1.6<br />
24 weeks d<br />
SSZ 2–3 g per day<br />
© Queen’s Printer and Controller <strong>of</strong> HMSO 2006. All rights reserved.<br />
Health Technology Assessment 2006; Vol. 10: No. 42<br />
Protocol 0881A1-308-EU/AU<br />
TEMPO: Klareskog et al., MTX (starting 7.5, escalating to 20 mg per week 228 53 6.8 2.3 64 86 1.7<br />
2004 110,127,128<br />
if any painful/swollen joint; mean 18 mg) + placebo<br />
Europe and Australia, multicentre, Etanercept 25 mg s.c. twice weekly + placebo 223 53 6.3 2.3 57 86 1.8<br />
double-blind Etanercept 25 mg s.c. twice weekly + MTX 231 53 6.8 2.3 62 86 1.8<br />
Duration <strong>of</strong> treatment and follow-up: (starting 7.5, escalating to 20 mg per week if any<br />
period one 52 weeks; period two painful/swollen joint; mean 18 mg)<br />
ongoing double-blind extension,<br />
with year 2 results reported<br />
continued<br />
33