Physical Principles of Electron Microscopy: An Introduction to TEM ...
Physical Principles of Electron Microscopy: An Introduction to TEM ...
Physical Principles of Electron Microscopy: An Introduction to TEM ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
94 Chapter 4<br />
-e -e<br />
-e<br />
-e<br />
+Ze<br />
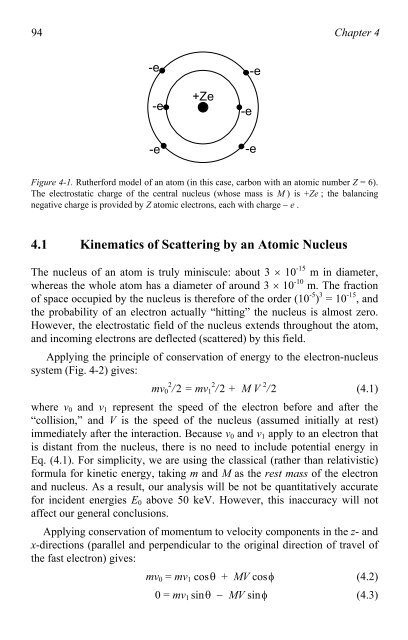
Figure 4-1. Rutherford model <strong>of</strong> an a<strong>to</strong>m (in this case, carbon with an a<strong>to</strong>mic number Z = 6).<br />
The electrostatic charge <strong>of</strong> the central nucleus (whose mass is M ) is +Ze ; the balancing<br />
negative charge is provided by Z a<strong>to</strong>mic electrons, each with charge � e .<br />
4.1 Kinematics <strong>of</strong> Scattering by an A<strong>to</strong>mic Nucleus<br />
The nucleus <strong>of</strong> an a<strong>to</strong>m is truly miniscule: about 3 � 10 -15 m in diameter,<br />
whereas the whole a<strong>to</strong>m has a diameter <strong>of</strong> around 3 � 10 -10 m. The fraction<br />
<strong>of</strong> space occupied by the nucleus is therefore <strong>of</strong> the order (10 -5 ) 3 = 10 -15 , and<br />
the probability <strong>of</strong> an electron actually “hitting” the nucleus is almost zero.<br />
However, the electrostatic field <strong>of</strong> the nucleus extends throughout the a<strong>to</strong>m,<br />
and incoming electrons are deflected (scattered) by this field.<br />
Applying the principle <strong>of</strong> conservation <strong>of</strong> energy <strong>to</strong> the electron-nucleus<br />
system (Fig. 4-2) gives:<br />
mv0 2 /2 = mv1 2 /2 + M V 2 /2 (4.1)<br />
where v0 and v1 represent the speed <strong>of</strong> the electron before and after the<br />
“collision,” and V is the speed <strong>of</strong> the nucleus (assumed initially at rest)<br />
immediately after the interaction. Because v0 and v1 apply <strong>to</strong> an electron that<br />
is distant from the nucleus, there is no need <strong>to</strong> include potential energy in<br />
Eq. (4.1). For simplicity, we are using the classical (rather than relativistic)<br />
formula for kinetic energy, taking m and M as the rest mass <strong>of</strong> the electron<br />
and nucleus. As a result, our analysis will be not be quantitatively accurate<br />
for incident energies E0 above 50 keV. However, this inaccuracy will not<br />
affect<br />
our general conclusions.<br />
Applying conservation <strong>of</strong> momentum <strong>to</strong> velocity components in the z- and<br />
x-directions (parallel and perpendicular <strong>to</strong> the original direction <strong>of</strong> travel <strong>of</strong><br />
the<br />
fast electron) gives:<br />
mv0 = mv1 cos� + MV cos� (4.2)<br />
0 = mv1 sin� � MV sin� (4.3)<br />
-e<br />
-e