Physical Principles of Electron Microscopy: An Introduction to TEM ...
Physical Principles of Electron Microscopy: An Introduction to TEM ...
Physical Principles of Electron Microscopy: An Introduction to TEM ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
The Transmission <strong>Electron</strong> Microscope 63<br />
only about 1800 K <strong>to</strong> provide adequate electron emission. The tip protrudes<br />
about 0.3 mm outside the hole in the Wehnelt, so an accelerating field exists<br />
at its surface, created by an extrac<strong>to</strong>r electrode biased positive with respect <strong>to</strong><br />
the tip. Because the tip is very sharp, electrons are emitted from a very small<br />
area, resulting in a relatively high current density (Je � 10 7 A/m 2 ) at the<br />
surface. Because the ZrO is easily poisoned by ambient gases, the Schottky<br />
source requires a vacuum substantially better than that <strong>of</strong> a LaB6 source.<br />
Field emission<br />
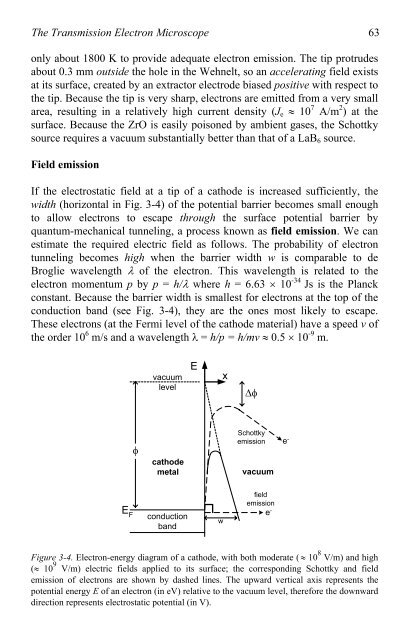
If the electrostatic field at a tip <strong>of</strong> a cathode is increased sufficiently, the<br />
width (horizontal in Fig. 3-4) <strong>of</strong> the potential barrier becomes small enough<br />
<strong>to</strong> allow electrons <strong>to</strong> escape through the surface potential barrier by<br />
quantum-mechanical tunneling, a process known as field emission. We can<br />
estimate the required electric field as follows. The probability <strong>of</strong> electron<br />
tunneling becomes high when the barrier width w is comparable <strong>to</strong> de<br />
Broglie wavelength � <strong>of</strong> the electron. This wavelength is related <strong>to</strong> the<br />
electron momentum p by p = h/� where h = 6.63 � 10 -34 Js is the Planck<br />
constant. Because the barrier width is smallest for electrons at the <strong>to</strong>p <strong>of</strong> the<br />
conduction band (see Fig. 3-4), they are the ones most likely <strong>to</strong> escape.<br />
These electrons (at the Fermi level <strong>of</strong> the cathode material) have a speed v <strong>of</strong><br />
the order 10 6 m/s and a wavelength � = h/p = h/mv � 0.5 � 10 -9 m.<br />
E F<br />
�<br />
vacuum<br />
level<br />
cathode<br />
metal<br />
conduction<br />
band<br />
E<br />
x<br />
Schottky<br />
emission<br />
vacuum<br />
field<br />
emission<br />
e- Figure 3-4. <strong>Electron</strong>-energy diagram <strong>of</strong> a cathode, with both moderate ( � 10 8 V/m) and high<br />
(� 10 9 V/m) electric fields applied <strong>to</strong> its surface; the corresponding Schottky and field<br />
emission <strong>of</strong> electrons are shown by dashed lines. The upward vertical axis represents the<br />
potential energy E <strong>of</strong> an electron (in eV) relative <strong>to</strong> the vacuum level, therefore the downward<br />
direction represents electrostatic potential (in V).<br />
w<br />
��<br />
e -