Physical Principles of Electron Microscopy: An Introduction to TEM ...
Physical Principles of Electron Microscopy: An Introduction to TEM ...
Physical Principles of Electron Microscopy: An Introduction to TEM ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
The Transmission <strong>Electron</strong> Microscope 59<br />
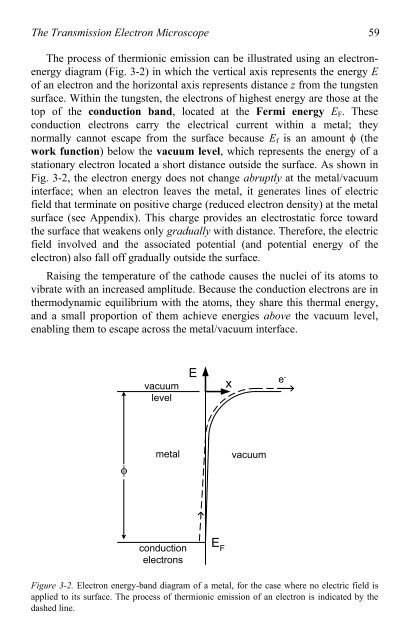
The process <strong>of</strong> thermionic emission can be illustrated using an electronenergy<br />
diagram (Fig. 3-2) in which the vertical axis represents the energy E<br />
<strong>of</strong> an electron and the horizontal axis represents distance z from the tungsten<br />
surface. Within the tungsten, the electrons <strong>of</strong> highest energy are those at the<br />
<strong>to</strong>p <strong>of</strong> the conduction band, located at the Fermi energy EF. These<br />
conduction electrons carry the electrical current within a metal; they<br />
normally cannot escape from the surface because Ef is an amount � (the<br />
work function) below the vacuum level, which represents the energy <strong>of</strong> a<br />
stationary electron located a short distance outside the surface. As shown in<br />
Fig. 3-2, the electron energy does not change abruptly at the metal/vacuum<br />
interface; when an electron leaves the metal, it generates lines <strong>of</strong> electric<br />
field that terminate on positive charge (reduced electron density) at the metal<br />
surface (see Appendix). This charge provides an electrostatic force <strong>to</strong>ward<br />
the surface that weakens only gradually with distance. Therefore, the electric<br />
field involved and the associated potential (and potential energy <strong>of</strong> the<br />
electron) also fall <strong>of</strong>f gradually outside the surface.<br />
Raising the temperature <strong>of</strong> the cathode causes the nuclei <strong>of</strong> its a<strong>to</strong>ms <strong>to</strong><br />
vibrate with an increased amplitude. Because the conduction electrons are in<br />
thermodynamic equilibrium with the a<strong>to</strong>ms, they share this thermal energy,<br />
and a small proportion <strong>of</strong> them achieve energies above the vacuum level,<br />
enabling them <strong>to</strong> escape across the metal/vacuum interface.<br />
�<br />
vacuum<br />
level<br />
metal<br />
conduction<br />
electrons<br />
E<br />
E F<br />
x<br />
vacuum<br />
Figure 3-2. <strong>Electron</strong> energy-band diagram <strong>of</strong> a metal, for the case where no electric field is<br />
applied <strong>to</strong> its surface. The process <strong>of</strong> thermionic emission <strong>of</strong> an electron is indicated by the<br />
dashed line.<br />
e -