Physical Principles of Electron Microscopy: An Introduction to TEM ...
Physical Principles of Electron Microscopy: An Introduction to TEM ...
Physical Principles of Electron Microscopy: An Introduction to TEM ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
<strong>TEM</strong> Specimens and Images 95<br />
v 0<br />
m<br />
M<br />
z<br />
(a) before (b) after<br />
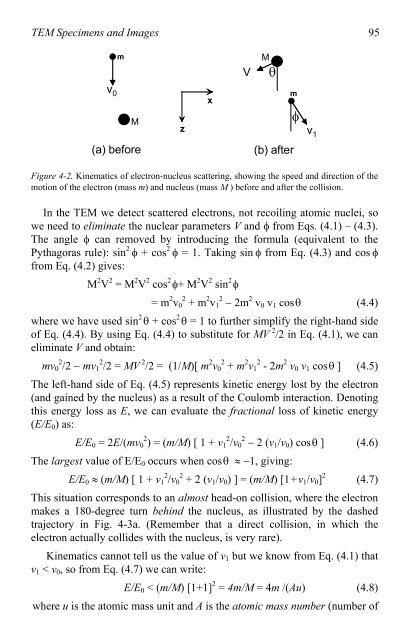
Figure 4-2. Kinematics <strong>of</strong> electron-nucleus scattering, showing the speed and direction <strong>of</strong> the<br />
motion <strong>of</strong> the electron (mass m) and nucleus (mass M ) before and after the collision.<br />
In the <strong>TEM</strong> we detect scattered electrons, not recoiling a<strong>to</strong>mic nuclei, so<br />
we need <strong>to</strong> eliminate the nuclear parameters V and � from Eqs. (4.1) – (4.3).<br />
The angle � can removed by introducing the formula (equivalent <strong>to</strong> the<br />
Pythagoras rule): sin 2 � + cos 2 � = 1. Taking sin � from Eq. (4.3) and cos �<br />
from<br />
Eq. (4.2) gives:<br />
M 2 V 2 = M 2 V 2 cos 2 �+ M 2 V 2 sin 2 �<br />
= m 2 v0 2 + m 2 v1 2 � 2m 2 v0 v1 cos� (4.4)<br />
where we have used sin 2 � + cos 2 � = 1 <strong>to</strong> further simplify the right-hand side<br />
<strong>of</strong> Eq. (4.4). By using Eq. (4.4) <strong>to</strong> substitute for MV 2 /2 in Eq. (4.1), we can<br />
eliminate<br />
V and obtain:<br />
mv0 2 /2 � mv1 2 /2 = MV 2 /2 = (1/M)[ m 2 v0 2 + m 2 v1 2 - 2m 2 v0 v1 cos� ] (4.5)<br />
The left-hand side <strong>of</strong> Eq. (4.5) represents kinetic energy lost by the electron<br />
(and gained by the nucleus) as a result <strong>of</strong> the Coulomb interaction. Denoting<br />
this energy loss as E, we can evaluate the fractional loss <strong>of</strong> kinetic energy<br />
( E/E0) as:<br />
E/E0 = 2E/(mv0 2 ) = (m/M) [ 1 + v1 2 /v0 2 � 2 (v1/v0) cos� ] (4.6)<br />
The largest value <strong>of</strong> E/E0 occurs when cos� ��1, giving:<br />
E/E0 � (m/M) [ 1 + v1 2 /v0 2 + 2 (v1/v0) ] = (m/M) [1+v1/v0] 2<br />
(4.7)<br />
This situation corresponds <strong>to</strong> an almost head-on collision, where the electron<br />
makes a 180-degree turn behind the nucleus, as illustrated by the dashed<br />
trajec<strong>to</strong>ry in Fig. 4-3a. (Remember that a direct collision, in which the<br />
electron actually collides with the nucleus, is very rare).<br />
Kinematics cannot tell us the value <strong>of</strong> v1 but we know from Eq. (4.1) that<br />
v1 < v0, so from Eq. (4.7) we can write:<br />
E/E0 < (m/M) [1+1] 2 = 4m/M = 4m /(Au) (4.8)<br />
where u is the a<strong>to</strong>mic mass unit and A is the a<strong>to</strong>mic mass number (number <strong>of</strong><br />
x<br />
V<br />
M<br />
�<br />
m<br />
�<br />
v 1