4(%3)3 - Ecole nationale supérieure de chimie de Montpellier
4(%3)3 - Ecole nationale supérieure de chimie de Montpellier
4(%3)3 - Ecole nationale supérieure de chimie de Montpellier
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
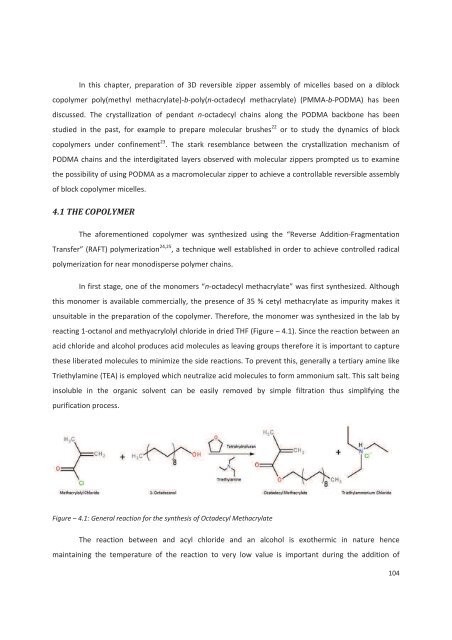
In this chapter, preparation of 3D reversible zipper assembly of micelles based on a diblockcopolymer poly(methyl methacrylate)-b-poly(n-octa<strong>de</strong>cyl methacrylate) (PMMA-b-PODMA) has beendiscussed. The crystallization of pendant n-octa<strong>de</strong>cyl chains along the PODMA backbone has beenstudied in the past, for example to prepare molecular brushes 22 or to study the dynamics of blockcopolymers un<strong>de</strong>r confinement 23 . The stark resemblance between the crystallization mechanism ofPODMA chains and the interdigitated layers observed with molecular zippers prompted us to examinethe possibility of using PODMA as a macromolecular zipper to achieve a controllable reversible assemblyof block copolymer micelles.4.1 THE COPOLYMERThe aforementioned copolymer was synthesized using the “Reverse Addition-FragmentationTransfer” (RAFT) polymerization 24,25 , a technique well established in or<strong>de</strong>r to achieve controlled radicalpolymerization for near monodisperse polymer chains.In first stage, one of the monomers “n-octa<strong>de</strong>cyl methacrylate” was first synthesized. Althoughthis monomer is available commercially, the presence of 35 % cetyl methacrylate as impurity makes itunsuitable in the preparation of the copolymer. Therefore, the monomer was synthesized in the lab byreacting 1-octanol and methyacrylolyl chlori<strong>de</strong> in dried THF (Figure – 4.1). Since the reaction between anacid chlori<strong>de</strong> and alcohol produces acid molecules as leaving groups therefore it is important to capturethese liberated molecules to minimize the si<strong>de</strong> reactions. To prevent this, generally a tertiary amine likeTriethylamine (TEA) is employed which neutralize acid molecules to form ammonium salt. This salt beinginsoluble in the organic solvent can be easily removed by simple filtration thus simplifying thepurification process.Figure – 4.1: General reaction for the synthesis of Octa<strong>de</strong>cyl MethacrylateThe reaction between and acyl chlori<strong>de</strong> and an alcohol is exothermic in nature hencemaintaining the temperature of the reaction to very low value is important during the addition of104