4(%3)3 - Ecole nationale supérieure de chimie de Montpellier
4(%3)3 - Ecole nationale supérieure de chimie de Montpellier
4(%3)3 - Ecole nationale supérieure de chimie de Montpellier
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
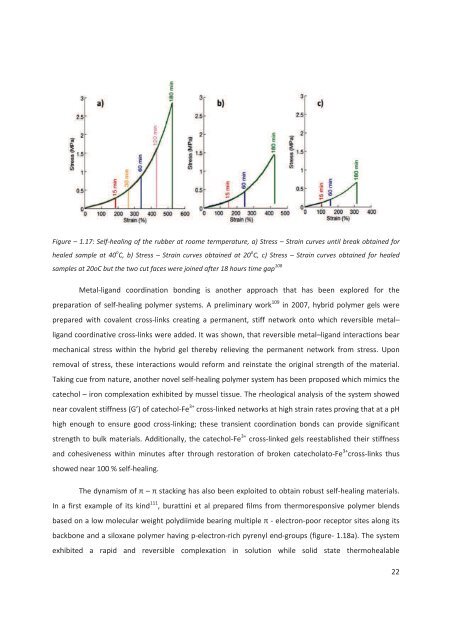
Figure – 1.17: Self-healing of the rubber at roome termperature, a) Stress – Strain curves until break obtained forhealed sample at 40 o C, b) Stress – Strain curves obtained at 20 o C, c) Stress – Strain curves obtained for healedsamples at 20oC but the two cut faces were joined after 18 hours time gap 108Metal-ligand coordination bonding is another approach that has been explored for thepreparation of self-healing polymer systems. A preliminary work 109 in 2007, hybrid polymer gels wereprepared with covalent cross-links creating a permanent, stiff network onto which reversible metal–ligand coordinative cross-links were ad<strong>de</strong>d. It was shown, that reversible metal–ligand interactions bearmechanical stress within the hybrid gel thereby relieving the permanent network from stress. Uponremoval of stress, these interactions would reform and reinstate the original strength of the material.Taking cue from nature, another novel self-healing polymer system has been proposed which mimics thecatechol – iron complexation exhibited by mussel tissue. The rheological analysis of the system showednear covalent stiffness (G’) of catechol-Fe 3+ cross-linked networks at high strain rates proving that at a pHhigh enough to ensure good cross-linking; these transient coordination bonds can provi<strong>de</strong> significantstrength to bulk materials. Additionally, the catechol-Fe 3+ cross-linked gels reestablished their stiffnessand cohesiveness within minutes after through restoration of broken catecholato-Fe 3+ cross-links thusshowed near 100 % self-healing.– been exploited to obtain robust self-healing materials.In a first example of its kind 111 , burattini et al prepared films from thermoresponsive polymer blends- electron-poor receptor sites along itsbackbone and a siloxane polymer having p-electron-rich pyrenyl end-groups (figure- 1.18a). The systemexhibited a rapid and reversible complexation in solution while solid state thermohealable22