4(%3)3 - Ecole nationale supérieure de chimie de Montpellier
4(%3)3 - Ecole nationale supérieure de chimie de Montpellier
4(%3)3 - Ecole nationale supérieure de chimie de Montpellier
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
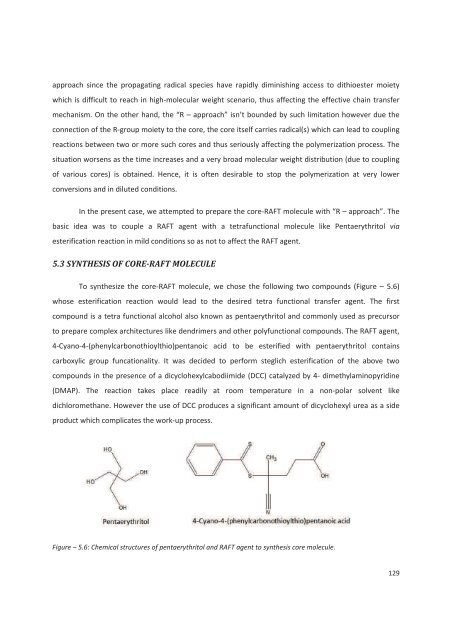
approach since the propagating radical species have rapidly diminishing access to dithioester moietywhich is difficult to reach in high-molecular weight scenario, thus affecting the effective chain transfermechanism. On the other hand, the “R – approach” isn’t boun<strong>de</strong>d by such limitation however due theconnection of the R-group moiety to the core, the core itself carries radical(s) which can lead to couplingreactions between two or more such cores and thus seriously affecting the polymerization process. Thesituation worsens as the time increases and a very broad molecular weight distribution (due to couplingof various cores) is obtained. Hence, it is often <strong>de</strong>sirable to stop the polymerization at very lowerconversions and in diluted conditions.In the present case, we attempted to prepare the core-RAFT molecule with “R – approach”. Thebasic i<strong>de</strong>a was to couple a RAFT agent with a tetrafunctional molecule like Pentaerythritol viaesterification reaction in mild conditions so as not to affect the RAFT agent.5.3 SYNTHESIS OF CORE-RAFT MOLECULETo synthesize the core-RAFT molecule, we chose the following two compounds (Figure – 5.6)whose esterification reaction would lead to the <strong>de</strong>sired tetra functional transfer agent. The firstcompound is a tetra functional alcohol also known as pentaerythritol and commonly used as precursorto prepare complex architectures like <strong>de</strong>ndrimers and other polyfunctional compounds. The RAFT agent,4-Cyano-4-(phenylcarbonothioylthio)pentanoic acid to be esterified with pentaerythritol containscarboxylic group funcationality. It was <strong>de</strong>ci<strong>de</strong>d to perform steglich esterification of the above twocompounds in the presence of a dicyclohexylcabodiimi<strong>de</strong> (DCC) catalyzed by 4- dimethylaminopyridine(DMAP). The reaction takes place readily at room temperature in a non-polar solvent likedichloromethane. However the use of DCC produces a significant amount of dicyclohexyl urea as a si<strong>de</strong>product which complicates the work-up process.Figure – 5.6: Chemical structures of pentaerythritol and RAFT agent to synthesis core molecule.129