4(%3)3 - Ecole nationale supérieure de chimie de Montpellier
4(%3)3 - Ecole nationale supérieure de chimie de Montpellier
4(%3)3 - Ecole nationale supérieure de chimie de Montpellier
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
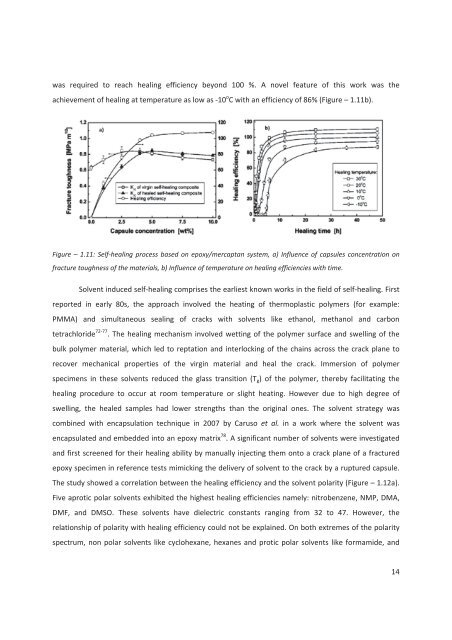
was required to reach healing efficiency beyond 100 %. A novel feature of this work was theachievement of healing at temperature as low as -10 o C with an efficiency of 86% (Figure – 1.11b).Figure – 1.11: Self-healing process based on epoxy/mercaptan system, a) Influence of capsules concentration onfracture toughness of the materials, b) Influence of temperature on healing efficiencies with time.Solvent induced self-healing comprises the earliest known works in the field of self-healing. Firstreported in early 80s, the approach involved the heating of thermoplastic polymers (for example:PMMA) and simultaneous sealing of cracks with solvents like ethanol, methanol and carbontetrachlori<strong>de</strong> 72-77 . The healing mechanism involved wetting of the polymer surface and swelling of thebulk polymer material, which led to reptation and interlocking of the chains across the crack plane torecover mechanical properties of the virgin material and heal the crack. Immersion of polymerspecimens in these solvents reduced the glass transition (T g ) of the polymer, thereby facilitating thehealing procedure to occur at room temperature or slight heating. However due to high <strong>de</strong>gree ofswelling, the healed samples had lower strengths than the original ones. The solvent strategy wascombined with encapsulation technique in 2007 by Caruso et al. in a work where the solvent wasencapsulated and embed<strong>de</strong>d into an epoxy matrix 78 . A significant number of solvents were investigatedand first screened for their healing ability by manually injecting them onto a crack plane of a fracture<strong>de</strong>poxy specimen in reference tests mimicking the <strong>de</strong>livery of solvent to the crack by a ruptured capsule.The study showed a correlation between the healing efficiency and the solvent polarity (Figure – 1.12a).Five aprotic polar solvents exhibited the highest healing efficiencies namely: nitrobenzene, NMP, DMA,DMF, and DMSO. These solvents have dielectric constants ranging from 32 to 47. However, therelationship of polarity with healing efficiency could not be explained. On both extremes of the polarityspectrum, non polar solvents like cyclohexane, hexanes and protic polar solvents like formami<strong>de</strong>, and14