4(%3)3 - Ecole nationale supérieure de chimie de Montpellier
4(%3)3 - Ecole nationale supérieure de chimie de Montpellier
4(%3)3 - Ecole nationale supérieure de chimie de Montpellier
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
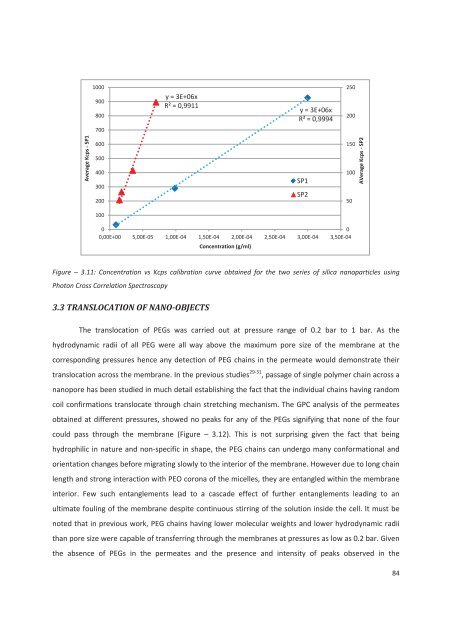
1000900800y = 3E+06xR² = 0,9911y = 3E+06xR² = 0,9994250200700Average Kcps - SP1600500400300200SP1SP215010050AVerage Kcps - SP2100000,00E+00 5,00E-05 1,00E-04 1,50E-04 2,00E-04 2,50E-04 3,00E-04 3,50E-04Concentration (g/ml)Figure – 3.11: Concentration vs Kcps calibration curve obtained for the two series of silica nanoparticles usingPhoton Cross Correlation Spectroscopy3.3 TRANSLOCATION OF NANO-OBJECTSThe translocation of PEGs was carried out at pressure range of 0.2 bar to 1 bar. As thehydrodynamic radii of all PEG were all way above the maximum pore size of the membrane at thecorresponding pressures hence any <strong>de</strong>tection of PEG chains in the permeate would <strong>de</strong>monstrate theirtranslocation across the membrane. In the previous studies 29-31 , passage of single polymer chain across ananopore has been studied in much <strong>de</strong>tail establishing the fact that the individual chains having randomcoil confirmations translocate through chain stretching mechanism. The GPC analysis of the permeatesobtained at different pressures, showed no peaks for any of the PEGs signifying that none of the fourcould pass through the membrane (Figure – 3.12). This is not surprising given the fact that beinghydrophilic in nature and non-specific in shape, the PEG chains can un<strong>de</strong>rgo many conformational andorientation changes before migrating slowly to the interior of the membrane. However due to long chainlength and strong interaction with PEO corona of the micelles, they are entangled within the membraneinterior. Few such entanglements lead to a casca<strong>de</strong> effect of further entanglements leading to anultimate fouling of the membrane <strong>de</strong>spite continuous stirring of the solution insi<strong>de</strong> the cell. It must benoted that in previous work, PEG chains having lower molecular weights and lower hydrodynamic radiithan pore size were capable of transferring through the membranes at pressures as low as 0.2 bar. Giventhe absence of PEGs in the permeates and the presence and intensity of peaks observed in the84