4(%3)3 - Ecole nationale supérieure de chimie de Montpellier
4(%3)3 - Ecole nationale supérieure de chimie de Montpellier
4(%3)3 - Ecole nationale supérieure de chimie de Montpellier
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
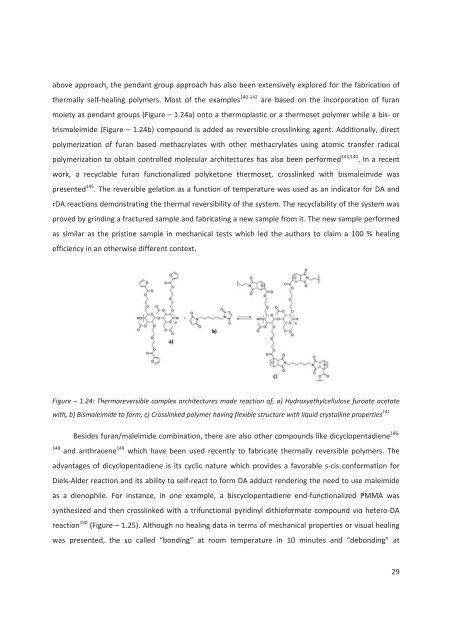
above approach, the pendant group approach has also been extensively explored for the fabrication ofthermally self-healing polymers. Most of the examples 140-142 are based on the incorporation of furanmoiety as pendant groups (Figure – 1.24a) onto a thermoplastic or a thermoset polymer while a bis- ortrismaleimi<strong>de</strong> (Figure – 1.24b) compound is ad<strong>de</strong>d as reversible crosslinking agent. Additionally, directpolymerization of furan based methacrylates with other methacrylates using atomic transfer radicalpolymerization to obtain controlled molecular architectures has also been performed 143,144 . In a recentwork, a recyclable furan functionalized polyketone thermoset, crosslinked with bismaleimi<strong>de</strong> waspresented 145 . The reversible gelation as a function of temperature was used as an indicator for DA andrDA reactions <strong>de</strong>monstrating the thermal reversibility of the system. The recyclability of the system wasproved by grinding a fractured sample and fabricating a new sample from it. The new sample performedas similar as the pristine sample in mechanical tests which led the authors to claim a 100 % healingefficiency in an otherwise different context.Figure – 1.24: Thermoreversible complex architectures ma<strong>de</strong> reaction of, a) Hydroxyethylcellulose furoate acetatewith, b) Bismaleimi<strong>de</strong> to form, c) Crosslinked polymer having flexible structure with liquid crystalline properties 141Besi<strong>de</strong>s furan/maleimi<strong>de</strong> combination, there are also other compounds like dicyclopentadiene 146-148 and anthracene 149 which have been used recently to fabricate thermally reversible polymers. Theadvantages of dicyclopentadiene is its cyclic nature which provi<strong>de</strong>s a favorable s-cis conformation forDiels-Al<strong>de</strong>r reaction and its ability to self-react to form DA adduct ren<strong>de</strong>ring the need to use maleimi<strong>de</strong>as a dienophile. For instance, in one example, a biscyclopentadiene end-functionalized PMMA wassynthesized and then crosslinked with a trifunctional pyridinyl dithioformate compound via hetero-DAreaction 150 (Figure – 1.25). Although no healing data in terms of mechanical properties or visual healingwas presented, the so called “bonding” at room temperature in 10 minutes and “<strong>de</strong>bonding” at29