4(%3)3 - Ecole nationale supérieure de chimie de Montpellier
4(%3)3 - Ecole nationale supérieure de chimie de Montpellier
4(%3)3 - Ecole nationale supérieure de chimie de Montpellier
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
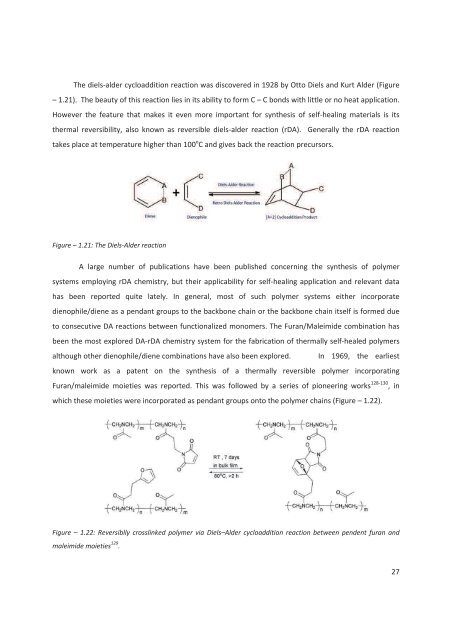
The diels-al<strong>de</strong>r cycloaddition reaction was discovered in 1928 by Otto Diels and Kurt Al<strong>de</strong>r (Figure– 1.21). The beauty of this reaction lies in its ability to form C – C bonds with little or no heat application.However the feature that makes it even more important for synthesis of self-healing materials is itsthermal reversibility, also known as reversible diels-al<strong>de</strong>r reaction (rDA). Generally the rDA reactiontakes place at temperature higher than 100 o C and gives back the reaction precursors.Figure – 1.21: The Diels-Al<strong>de</strong>r reactionA large number of publications have been published concerning the synthesis of polymersystems employing rDA chemistry, but their applicability for self-healing application and relevant datahas been reported quite lately. In general, most of such polymer systems either incorporatedienophile/diene as a pendant groups to the backbone chain or the backbone chain itself is formed dueto consecutive DA reactions between functionalized monomers. The Furan/Maleimi<strong>de</strong> combination hasbeen the most explored DA-rDA chemistry system for the fabrication of thermally self-healed polymersalthough other dienophile/diene combinations have also been explored. In 1969, the earliestknown work as a patent on the synthesis of a thermally reversible polymer incorporatingFuran/maleimi<strong>de</strong> moieties was reported. This was followed by a series of pioneering works 128-130 , inwhich these moieties were incorporated as pendant groups onto the polymer chains (Figure – 1.22).Figure – 1.22: Reversiblly crosslinked polymer via Diels–Al<strong>de</strong>r cycloaddition reaction between pen<strong>de</strong>nt furan andmaleimi<strong>de</strong> moieties 129 .27