Insomnia Insomnia
Insomnia Insomnia
Insomnia Insomnia
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
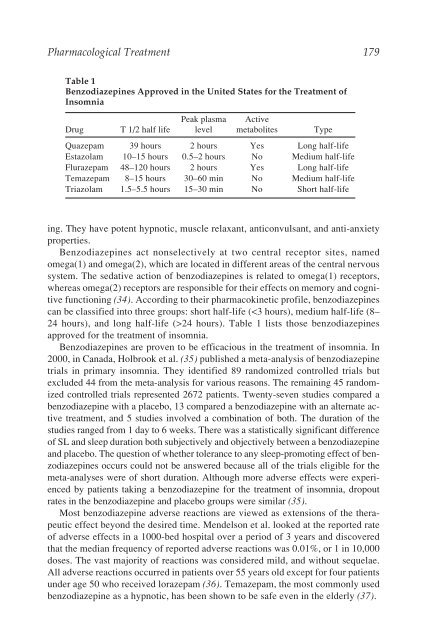
Pharmacological Treatment 179<br />
Table 1<br />
Benzodiazepines Approved in the United States for the Treatment of<br />
<strong>Insomnia</strong><br />
Peak plasma Active<br />
Drug T 1/2 half life level metabolites Type<br />
Quazepam 39 hours 2 hours Yes Long half-life<br />
Estazolam 10–15 hours 0.5–2 hours No Medium half-life<br />
Flurazepam 48–120 hours 2 hours Yes Long half-life<br />
Temazepam 8–15 hours 30–60 min No Medium half-life<br />
Triazolam 1.5–5.5 hours 15–30 min No Short half-life<br />
ing. They have potent hypnotic, muscle relaxant, anticonvulsant, and anti-anxiety<br />
properties.<br />
Benzodiazepines act nonselectively at two central receptor sites, named<br />
omega(1) and omega(2), which are located in different areas of the central nervous<br />
system. The sedative action of benzodiazepines is related to omega(1) receptors,<br />
whereas omega(2) receptors are responsible for their effects on memory and cognitive<br />
functioning (34). According to their pharmacokinetic profile, benzodiazepines<br />
can be classified into three groups: short half-life (24 hours). Table 1 lists those benzodiazepines<br />
approved for the treatment of insomnia.<br />
Benzodiazepines are proven to be efficacious in the treatment of insomnia. In<br />
2000, in Canada, Holbrook et al. (35) published a meta-analysis of benzodiazepine<br />
trials in primary insomnia. They identified 89 randomized controlled trials but<br />
excluded 44 from the meta-analysis for various reasons. The remaining 45 randomized<br />
controlled trials represented 2672 patients. Twenty-seven studies compared a<br />
benzodiazepine with a placebo, 13 compared a benzodiazepine with an alternate active<br />
treatment, and 5 studies involved a combination of both. The duration of the<br />
studies ranged from 1 day to 6 weeks. There was a statistically significant difference<br />
of SL and sleep duration both subjectively and objectively between a benzodiazepine<br />
and placebo. The question of whether tolerance to any sleep-promoting effect of benzodiazepines<br />
occurs could not be answered because all of the trials eligible for the<br />
meta-analyses were of short duration. Although more adverse effects were experienced<br />
by patients taking a benzodiazepine for the treatment of insomnia, dropout<br />
rates in the benzodiazepine and placebo groups were similar (35).<br />
Most benzodiazepine adverse reactions are viewed as extensions of the therapeutic<br />
effect beyond the desired time. Mendelson et al. looked at the reported rate<br />
of adverse effects in a 1000-bed hospital over a period of 3 years and discovered<br />
that the median frequency of reported adverse reactions was 0.01%, or 1 in 10,000<br />
doses. The vast majority of reactions was considered mild, and without sequelae.<br />
All adverse reactions occurred in patients over 55 years old except for four patients<br />
under age 50 who received lorazepam (36). Temazepam, the most commonly used<br />
benzodiazepine as a hypnotic, has been shown to be safe even in the elderly (37).