Insomnia Insomnia
Insomnia Insomnia
Insomnia Insomnia
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Pharmacological Treatment 181<br />
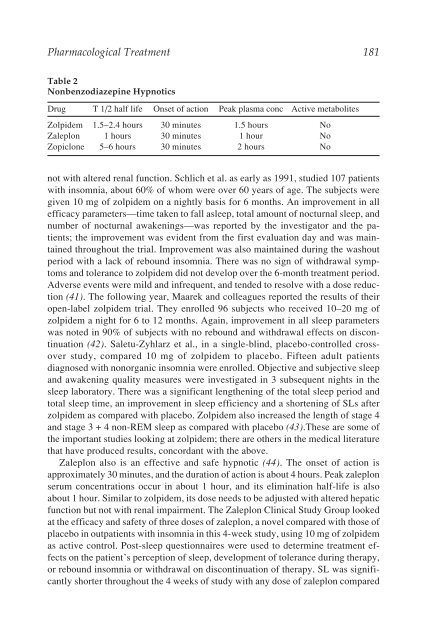
Table 2<br />
Nonbenzodiazepine Hypnotics<br />
Drug T 1/2 half life Onset of action Peak plasma conc Active metabolites<br />
Zolpidem 1.5–2.4 hours 30 minutes 1.5 hours No<br />
Zaleplon 1 hours 30 minutes 1 hour No<br />
Zopiclone 5–6 hours 30 minutes 2 hours No<br />
not with altered renal function. Schlich et al. as early as 1991, studied 107 patients<br />
with insomnia, about 60% of whom were over 60 years of age. The subjects were<br />
given 10 mg of zolpidem on a nightly basis for 6 months. An improvement in all<br />
efficacy parameters—time taken to fall asleep, total amount of nocturnal sleep, and<br />
number of nocturnal awakenings—was reported by the investigator and the patients;<br />
the improvement was evident from the first evaluation day and was maintained<br />
throughout the trial. Improvement was also maintained during the washout<br />
period with a lack of rebound insomnia. There was no sign of withdrawal symptoms<br />
and tolerance to zolpidem did not develop over the 6-month treatment period.<br />
Adverse events were mild and infrequent, and tended to resolve with a dose reduction<br />
(41). The following year, Maarek and colleagues reported the results of their<br />
open-label zolpidem trial. They enrolled 96 subjects who received 10–20 mg of<br />
zolpidem a night for 6 to 12 months. Again, improvement in all sleep parameters<br />
was noted in 90% of subjects with no rebound and withdrawal effects on discontinuation<br />
(42). Saletu-Zyhlarz et al., in a single-blind, placebo-controlled crossover<br />
study, compared 10 mg of zolpidem to placebo. Fifteen adult patients<br />
diagnosed with nonorganic insomnia were enrolled. Objective and subjective sleep<br />
and awakening quality measures were investigated in 3 subsequent nights in the<br />
sleep laboratory. There was a significant lengthening of the total sleep period and<br />
total sleep time, an improvement in sleep efficiency and a shortening of SLs after<br />
zolpidem as compared with placebo. Zolpidem also increased the length of stage 4<br />
and stage 3 + 4 non-REM sleep as compared with placebo (43).These are some of<br />
the important studies looking at zolpidem; there are others in the medical literature<br />
that have produced results, concordant with the above.<br />
Zaleplon also is an effective and safe hypnotic (44). The onset of action is<br />
approximately 30 minutes, and the duration of action is about 4 hours. Peak zaleplon<br />
serum concentrations occur in about 1 hour, and its elimination half-life is also<br />
about 1 hour. Similar to zolpidem, its dose needs to be adjusted with altered hepatic<br />
function but not with renal impairment. The Zaleplon Clinical Study Group looked<br />
at the efficacy and safety of three doses of zaleplon, a novel compared with those of<br />
placebo in outpatients with insomnia in this 4-week study, using 10 mg of zolpidem<br />
as active control. Post-sleep questionnaires were used to determine treatment effects<br />
on the patient’s perception of sleep, development of tolerance during therapy,<br />
or rebound insomnia or withdrawal on discontinuation of therapy. SL was significantly<br />
shorter throughout the 4 weeks of study with any dose of zaleplon compared