download pdf - Institut für Umweltphysik - Ruprecht-Karls-Universität ...
download pdf - Institut für Umweltphysik - Ruprecht-Karls-Universität ...
download pdf - Institut für Umweltphysik - Ruprecht-Karls-Universität ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
102 CHAPTER 2. ATMOSPHERE AND REMOTE SENSING<br />
2.6.4 Impact of reactive bromine chemistry in the troposphere<br />
Participating scientists Roland von Glasow, R. von Kuhlmann (MPI-C), M. G. Lawrence (MPI-<br />
C), U. Platt, and P. J. Crutzen (MPI-C, SIO)<br />
Abstract The potential impact of 0.5 - 2 pmol mol −1 BrO on the photochemistry in the free<br />
troposphere was investigated with the help of a global three-dimensional transport model. Annual<br />
zonal mean ozone mixing ratios are reduced by up to 20 % pointing to a potentially very significant<br />
ozone loss process that has been ignored so far.<br />
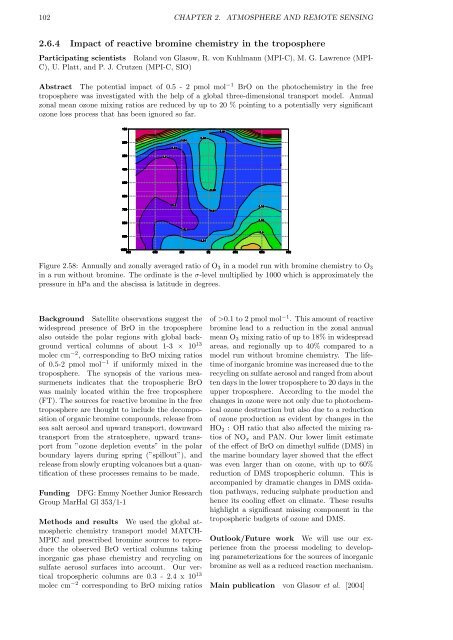
Figure 2.58: Annually and zonally averaged ratio of O3 in a model run with bromine chemistry to O3<br />
in a run without bromine. The ordinate is the σ-level multiplied by 1000 which is approximately the<br />
pressure in hPa and the abscissa is latitude in degrees.<br />
Background Satellite observations suggest the<br />
widespread presence of BrO in the troposphere<br />
also outside the polar regions with global background<br />
vertical columns of about 1-3 × 10 13<br />
molec cm −2 , corresponding to BrO mixing ratios<br />
of 0.5-2 pmol mol −1 if uniformly mixed in the<br />
troposphere. The synopsis of the various measurmenets<br />
indicates that the tropospheric BrO<br />
was mainly located within the free troposphere<br />
(FT). The sources for reactive bromine in the free<br />
troposphere are thought to include the decomposition<br />
of organic bromine compounds, release from<br />
sea salt aerosol and upward transport, downward<br />
transport from the stratosphere, upward transport<br />
from ”ozone depletion events” in the polar<br />
boundary layers during spring (”spillout”), and<br />
release from slowly erupting volcanoes but a quantification<br />
of these processes remains to be made.<br />
Funding DFG: Emmy Noether Junior Research<br />
Group MarHal Gl 353/1-1<br />
Methods and results We used the global atmospheric<br />
chemistry transport model MATCH-<br />
MPIC and prescribed bromine sources to reproduce<br />
the observed BrO vertical columns taking<br />
inorganic gas phase chemistry and recycling on<br />
sulfate aerosol surfaces into account. Our vertical<br />
tropospheric columns are 0.3 - 2.4 x 10 13<br />
molec cm −2 corresponding to BrO mixing ratios<br />
of >0.1 to 2 pmol mol −1 . This amount of reactive<br />
bromine lead to a reduction in the zonal annual<br />
mean O3 mixing ratio of up to 18% in widespread<br />
areas, and regionally up to 40% compared to a<br />
model run without bromine chemistry. The lifetime<br />
of inorganic bromine was increased due to the<br />
recycling on sulfate aerosol and ranged from about<br />
ten days in the lower troposphere to 20 days in the<br />
upper troposphere. According to the model the<br />
changes in ozone were not only due to photochemical<br />
ozone destruction but also due to a reduction<br />
of ozone production as evident by changes in the<br />
HO2 : OH ratio that also affected the mixing ratios<br />
of NOx and PAN. Our lower limit estimate<br />
of the effect of BrO on dimethyl sulfide (DMS) in<br />
the marine boundary layer showed that the effect<br />
was even larger than on ozone, with up to 60%<br />
reduction of DMS tropospheric column. This is<br />
accompanied by dramatic changes in DMS oxidation<br />
pathways, reducing sulphate production and<br />
hence its cooling effect on climate. These results<br />
highlight a significant missing component in the<br />
tropospheric budgets of ozone and DMS.<br />
Outlook/Future work We will use our experience<br />
from the process modeling to developing<br />
parameterizations for the sources of inorganic<br />
bromine as well as a reduced reaction mechanism.<br />
Main publication von Glasow et al. [2004]