Dissertation
Dissertation
Dissertation
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
|3.2 Bisphenanthroline: A Suitable Molecular Bridge?|<br />
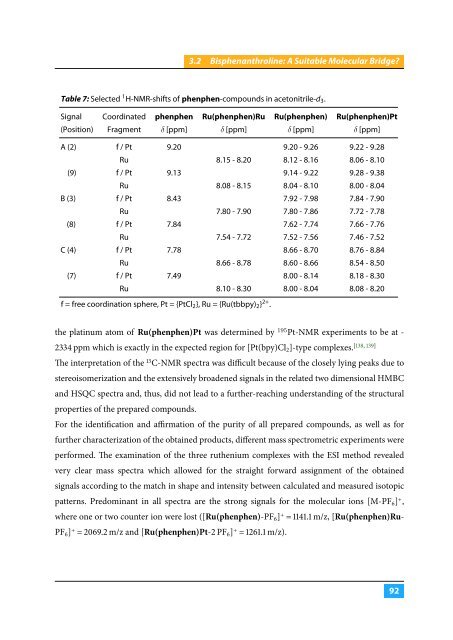
Table 7: Selected 1 H-NMR-shifts of phenphen-compounds in acetonitrile-d 3 .<br />
Signal Coordinated phenphen Ru(phenphen)Ru Ru(phenphen) Ru(phenphen)Pt<br />
(Position) Fragment δ [ppm] δ [ppm] δ [ppm] δ [ppm]<br />
A (2) f / Pt 9.20 9.20 - 9.26 9.22 - 9.28<br />
Ru 8.15 - 8.20 8.12 - 8.16 8.06 - 8.10<br />
A (9) f / Pt 9.13 9.14 - 9.22 9.28 - 9.38<br />
Ru 8.08 - 8.15 8.04 - 8.10 8.00 - 8.04<br />
B (3) f / Pt 8.43 7.92 - 7.98 7.84 - 7.90<br />
Ru 7.80 - 7.90 7.80 - 7.86 7.72 - 7.78<br />
B (8) f / Pt 7.84 7.62 - 7.74 7.66 - 7.76<br />
Ru 7.54 - 7.72 7.52 - 7.56 7.46 - 7.52<br />
C (4) f / Pt 7.78 8.66 - 8.70 8.76 - 8.84<br />
Ru 8.66 - 8.78 8.60 - 8.66 8.54 - 8.50<br />
C (7) f / Pt 7.49 8.00 - 8.14 8.18 - 8.30<br />
Ru 8.10 - 8.30 8.00 - 8.04 8.08 - 8.20<br />
f = free coordination sphere, Pt = {PtCl 2 }, Ru = {Ru(tbbpy) 2 } 2+ .<br />
the platinum atom of Ru(phenphen)Pt was determined by 195 Pt-NMR experiments to be at -<br />
2334 ppm which is exactly in the expected region for [Pt(bpy)Cl 2 ]-type complexes.<br />
[138, 139]<br />
The interpretation of the 13 C-NMR spectra was difficult because of the closely lying peaks due to<br />
stereoisomerization and the extensively broadened signals in the related two dimensional HMBC<br />
and HSQC spectra and, thus, did not lead to a further-reaching understanding of the structural<br />
properties of the prepared compounds.<br />
For the identification and affirmation of the purity of all prepared compounds, as well as for<br />
further characterization of the obtained products, different mass spectrometric experiments were<br />
performed. The examination of the three ruthenium complexes with the ESI method revealed<br />
very clear mass spectra which allowed for the straight forward assignment of the obtained<br />
signals according to the match in shape and intensity between calculated and measured isotopic<br />
patterns. Predominant in all spectra are the strong signals for the molecular ions [M-PF 6 ] + ,<br />
where one or two counter ion were lost ([Ru(phenphen)-PF 6 ] + = 1141.1 m/z, [Ru(phenphen)Ru-<br />
PF 6 ] + = 2069.2 m/z and [Ru(phenphen)Pt-2 PF 6 ] + = 1261.1 m/z).<br />
|92|