Dissertation
Dissertation
Dissertation
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
|6.1 Synthesis of the Organic Ligands|<br />
9<br />
8 N<br />
7 N<br />
6<br />
10<br />
5<br />
11<br />
4<br />
CH 2<br />
Ar<br />
1<br />
H-NMR (CDCl 3 , 400 MHz): δ = 9.168 (dd, 2H (6,9) , 3 J = 4.6 Hz, 4 J =<br />
1.6 Hz), 9.039 (dd, 2H (9,6) , 3 J = 4.6 Hz, 4 J = 1.6 Hz), 8.999 (dd, 2H (4,11) ,<br />
N 1<br />
2<br />
N<br />
3<br />
bip<br />
Ar<br />
Ar<br />
3<br />
J = 8.2 Hz, 4 J = 1.6 Hz), 8.191 (dd, 2H (11,4) , 3 J = 8.2 Hz, 4 J = 1.6 Hz),<br />
8.020 (s, 1H (2)) , 7.729 (dd, 2H (5,10) , 3 J = 8.2 Hz, 3 J = 4.4 Hz), 7.413 (dd,<br />
2H (5,10) , 3 J = 8.2 Hz, 3 J = 4.4 Hz), 7.3 (m, 3H (Ar) ), 7.1 (m, 2H (Ar) ), 7.3 (m,<br />
3H (Ar) ), 5.794 (s, 2H (-CH2) ) ppm. 13 C-NMR (CDCl 3 , 400 MHz): δ = 149.00 (1C (6,9) ), 148.06 (1C (9,6) ),<br />
144.68 (1C (3”,11’) ), 144.15 (1C (11’,3”) ), 143.54 (1C (2) ), 137.67 (1C (3’,11”) ), 134.99 (1C (C1-Ar) ), 130.48 (1C (4,11) ),<br />
129.41 (2C (Ar) ), 128.74 (1C (C4-Ar) ), 128.58 (1C (11,4) ), 126.00 (2C (Ar) ), 124.38 (1C (11”,3’) ), 124.35 (1C (7’,7”) ),<br />
123.68 (1C (5,10) ), 122.59 (1C (10,5) ), 119.58 (1C (7”,7’) ), 51.29 (1C (CH2 )) ppm.<br />
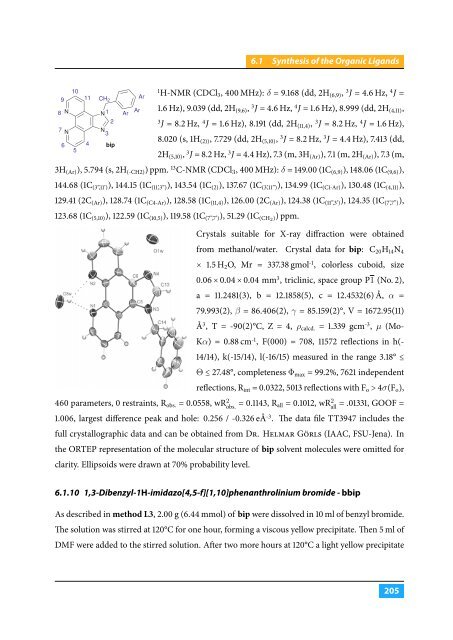
Crystals suitable for X-ray diffraction were obtained<br />
from methanol/water. Crystal data for bip: C 20 H 14 N 4<br />
× 1.5 H 2 O, Mr = 337.38 gmol -1 , colorless cuboid, size<br />
0.06 × 0.04 × 0.04 mm 3 , triclinic, space group P1 (No. 2),<br />
a = 11.2481(3), b = 12.1858(5), c = 12.4532(6) Å, α =<br />
79.993(2), β = 86.406(2), γ = 85.159(2)°, V = 1672.95(11)<br />
Å 3 , T = -90(2)°C, Z = 4, ρ calcd. = 1.339 gcm -3 , µ (Mo-<br />
Kα) = 0.88 cm -1 , F(000) = 708, 11572 reflections in h(-<br />
14/14), k(-15/14), l(-16/15) measured in the range 3.18° ≤<br />
Θ ≤ 27.48°, completeness Φ max = 99.2%, 7621 independent<br />
reflections, R int = 0.0322, 5013 reflections with F o > 4σ(F o ),<br />
460 parameters, 0 restraints, R obs. = 0.0558, wR 2 obs. = 0.1143, R all = 0.1012, wR 2 all<br />
= .01331, GOOF =<br />
1.006, largest difference peak and hole: 0.256 / -0.326 eÅ -3 . The data file TT3947 includes the<br />
full crystallographic data and can be obtained from Dr. Helmar Görls (IAAC, FSU-Jena). In<br />
the ORTEP representation of the molecular structure of bip solvent molecules were omitted for<br />
clarity. Ellipsoids were drawn at 70% probability level.<br />
6.1.10 1,3-Dibenzyl-1H-imidazo[4,5-f][1,10]phenanthrolinium bromide - bbip<br />
As described in method L3, 2.00 g (6.44 mmol) of bip were dissolved in 10 ml of benzyl bromide.<br />
The solution was stirred at 120°C for one hour, forming a viscous yellow precipitate. Then 5 ml of<br />
DMF were added to the stirred solution. After two more hours at 120°C a light yellow precipitate<br />
|205|