Dissertation
Dissertation
Dissertation
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
|1.9 Multicomponent Systems from Fundamental Building Blocks|<br />
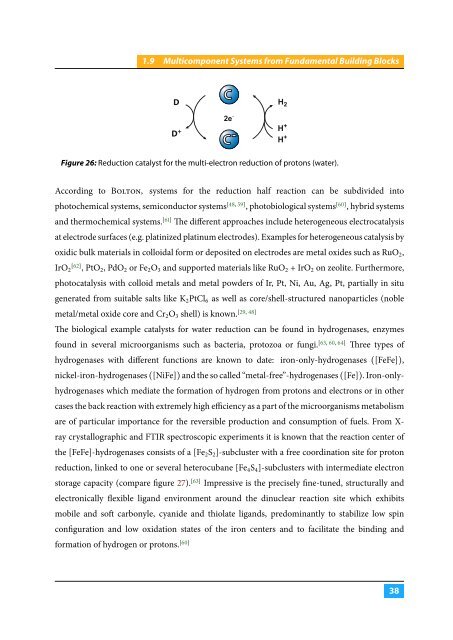
D<br />
C<br />
H 2<br />
D +<br />
2e -<br />
C - H +<br />
H +<br />
Figure 26: Reduction catalyst for the multi-electron reduction of protons (water).<br />
According to Bolton, systems for the reduction half reaction can be subdivided into<br />
photochemical systems, semiconductor systems [48, 59] , photobiological systems [60] , hybrid systems<br />
and thermochemical systems. [61] The different approaches include heterogeneous electrocatalysis<br />
at electrode surfaces (e.g. platinized platinum electrodes). Examples for heterogeneous catalysis by<br />
oxidic bulk materials in colloidal form or deposited on electrodes are metal oxides such as RuO 2 ,<br />
IrO 2<br />
[62]<br />
, PtO 2 , PdO 2 or Fe 2 O 3 and supported materials like RuO 2 + IrO 2 on zeolite. Furthermore,<br />
photocatalysis with colloid metals and metal powders of Ir, Pt, Ni, Au, Ag, Pt, partially in situ<br />
generated from suitable salts like K 2 PtCl 6 as well as core/shell-structured nanoparticles (noble<br />
metal/metal oxide core and Cr 2 O 3 shell) is known.<br />
[29, 48]<br />
The biological example catalysts for water reduction can be found in hydrogenases, enzymes<br />
found in several microorganisms such as bacteria, protozoa or fungi. [63, 60, 64] Three types of<br />
hydrogenases with different functions are known to date: iron-only-hydrogenases ([FeFe]),<br />
nickel-iron-hydrogenases ([NiFe]) and the so called “metal-free”-hydrogenases ([Fe]). Iron-onlyhydrogenases<br />
which mediate the formation of hydrogen from protons and electrons or in other<br />
cases the back reaction with extremely high efficiency as a part of the microorganisms metabolism<br />
are of particular importance for the reversible production and consumption of fuels. From X-<br />
ray crystallographic and FTIR spectroscopic experiments it is known that the reaction center of<br />
the [FeFe]-hydrogenases consists of a [Fe 2 S 2 ]-subcluster with a free coordination site for proton<br />
reduction, linked to one or several heterocubane [Fe 4 S 4 ]-subclusters with intermediate electron<br />
storage capacity (compare figure 27). [63] Impressive is the precisely fine-tuned, structurally and<br />
electronically flexible ligand environment around the dinuclear reaction site which exhibits<br />
mobile and soft carbonyle, cyanide and thiolate ligands, predominantly to stabilize low spin<br />
configuration and low oxidation states of the iron centers and to facilitate the binding and<br />
formation of hydrogen or protons. [60] |38|