Dissertation
Dissertation
Dissertation
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
|3.3 NN-NHC-Ligand bbip: Toward Second Generation Catalysts|<br />
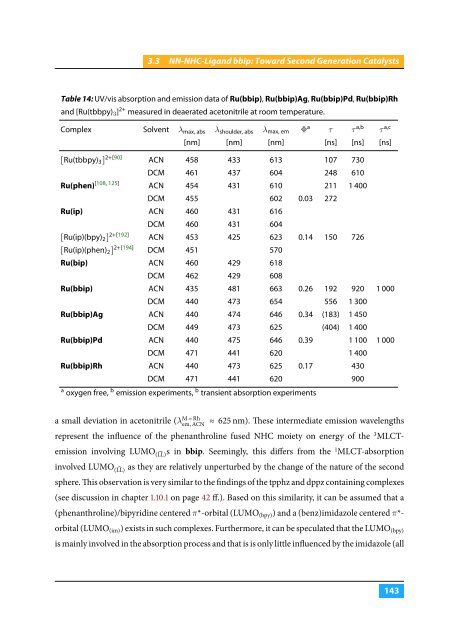
Table 14: UV/vis absorption and emission data of Ru(bbip), Ru(bbip)Ag, Ru(bbip)Pd, Ru(bbip)Rh<br />
and [Ru(tbbpy) 3 ] 2+ measured in deaerated acetonitrile at room temperature.<br />
Complex Solvent λ max, abs λ shoulder, abs λ max, em Φ a τ τ a,b τ a,c<br />
[nm] [nm] [nm] [ns] [ns] [ns]<br />
[Ru(tbbpy) 3 ] 2+[90] ACN 458 433 613 107 730<br />
DCM 461 437 604 248 610<br />
Ru(phen) [108, 125] ACN 454 431 610 211 1 400<br />
DCM 455 602 0.03 272<br />
Ru(ip) ACN 460 431 616<br />
DCM 460 431 604<br />
[Ru(ip)(bpy) 2 ] 2+[192] ACN 453 425 623 0.14 150 726<br />
[Ru(ip)(phen) 2 ] 2+[194] DCM 451 570<br />
Ru(bip) ACN 460 429 618<br />
DCM 462 429 608<br />
Ru(bbip) ACN 435 481 663 0.26 192 920 1 000<br />
DCM 440 473 654 556 1 300<br />
Ru(bbip)Ag ACN 440 474 646 0.34 (183) 1 450<br />
DCM 449 473 625 (404) 1 400<br />
Ru(bbip)Pd ACN 440 475 646 0.39 1 100 1 000<br />
DCM 471 441 620 1 400<br />
Ru(bbip)Rh ACN 440 473 625 0.17 430<br />
DCM 471 441 620 900<br />
a oxygen free, b emission experiments, b transient absorption experiments<br />
a small deviation in acetonitrile (λ M = Rh<br />
em, ACN<br />
≈ 625 nm). These intermediate emission wavelengths<br />
represent the influence of the phenanthroline fused NHC moiety on energy of the 3 MLCTemission<br />
involving LUMO ( ̂LL)s in bbip. Seemingly, this differs from the 1 MLCT-absorption<br />
involved LUMO ( ̂LL) as they are relatively unperturbed by the change of the nature of the second<br />
sphere. This observation is very similar to the findings of the tpphz and dppz containing complexes<br />
(see discussion in chapter 1.10.1 on page 42 ff.). Based on this similarity, it can be assumed that a<br />
(phenanthroline)/bipyridine centered π*-orbital (LUMO (bpy) ) and a (benz)imidazole centered π*-<br />
orbital (LUMO (im) ) exists in such complexes. Furthermore, it can be speculated that the LUMO (bpy)<br />
is mainly involved in the absorption process and that is is only little influenced by the imidazole (all<br />
|143|