Dissertation
Dissertation
Dissertation
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
|6.1 Synthesis of the Organic Ligands|<br />
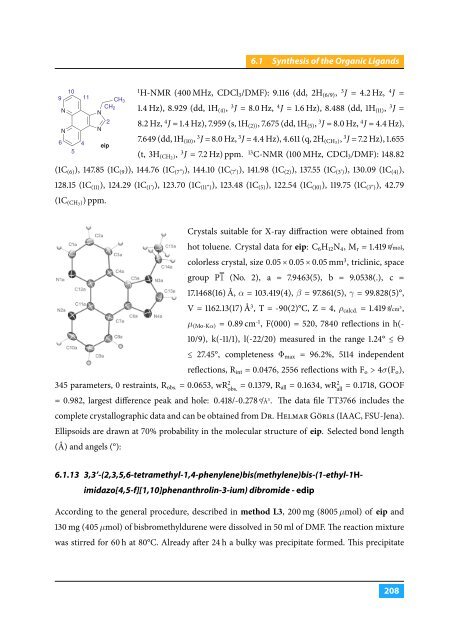
10<br />
9<br />
N<br />
N<br />
6<br />
5<br />
4<br />
11<br />
CH 3<br />
CH 2<br />
N<br />
2<br />
N<br />
eip<br />
1<br />
H-NMR (400 MHz, CDCl 3 /DMF): 9.116 (dd, 2H (6/9) , 3 J = 4.2 Hz, 4 J =<br />
1.4 Hz), 8.929 (dd, 1H (4) , 3 J = 8.0 Hz, 4 J = 1.6 Hz), 8.488 (dd, 1H (11) , 3 J =<br />
8.2 Hz, 4 J = 1.4 Hz), 7.959 (s, 1H (2)) , 7.675 (dd, 1H (5) , 3 J = 8.0 Hz, 4 J = 4.4 Hz),<br />
7.649 (dd, 1H (10) , 3 J = 8.0 Hz, 3 J = 4.4 Hz), 4.611 (q, 2H (CH3 ), 3 J = 7.2 Hz), 1.655<br />
(t, 3H (CH2 ), 3 J = 7.2 Hz) ppm.<br />
13<br />
C-NMR (100 MHz, CDCl 3 /DMF): 148.82<br />
(1C (6) ), 147.85 (1C (9 )), 144.76 (1C (7”) ), 144.10 (1C (7’) ), 141.98 (1C (2) ), 137.55 (1C (3’) ), 130.09 (1C (4) ),<br />
128.15 (1C (11) ), 124.29 (1C (1’) ), 123.70 (1C (11”) ), 123.48 (1C (5) ), 122.54 (1C (10) ), 119.75 (1C (3”) ), 42.79<br />
(1C (CH3 )) ppm.<br />
Crystals suitable for X-ray diffraction were obtained from<br />
hot toluene. Crystal data for eip: C 6 H 12 N 4 , M r = 1.419 g /mol,<br />
colorless crystal, size 0.05 × 0.05 × 0.05 mm 3 , triclinic, space<br />
group P1 (No. 2), a = 7.9463(5), b = 9.0538(.), c =<br />
17.1468(16) Å, α = 103.419(4), β = 97.861(5), γ = 99.828(5)°,<br />
V = 1162.13(17) Å 3 , T = -90(2)°C, Z = 4, ρ calcd. = 1.419 g /cm 3 ,<br />
µ (Mo-Kα) = 0.89 cm -1 , F(000) = 520, 7840 reflections in h(-<br />
10/9), k(-11/1), l(-22/20) measured in the range 1.24° ≤ Θ<br />
≤ 27.45°, completeness Φ max<br />
= 96.2%, 5114 independent<br />
reflections, R int = 0.0476, 2556 reflections with F o > 4σ(F o ),<br />
345 parameters, 0 restraints, R obs. = 0.0653, wR 2 obs.<br />
= 0.1379, R all = 0.1634, wR 2 all<br />
= 0.1718, GOOF<br />
= 0.982, largest difference peak and hole: 0.418/-0.278 e /Å 3 . The data file TT3766 includes the<br />
complete crystallographic data and can be obtained from Dr. Helmar Görls (IAAC, FSU-Jena).<br />
Ellipsoids are drawn at 70% probability in the molecular structure of eip. Selected bond length<br />
(Å) and angels (°):<br />
6.1.13 3,3’-(2,3,5,6-tetramethyl-1,4-phenylene)bis(methylene)bis-(1-ethyl-1Himidazo[4,5-f][1,10]phenanthrolin-3-ium)<br />
dibromide - edip<br />
According to the general procedure, described in method L3, 200 mg (8005 µmol) of eip and<br />
130 mg (405 µmol) of bisbromethyldurene were dissolved in 50 ml of DMF. The reaction mixture<br />
was stirred for 60 h at 80°C. Already after 24 h a bulky was precipitate formed. This precipitate<br />
|208|