Dissertation
Dissertation
Dissertation
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
|6.1 Synthesis of the Organic Ligands|<br />
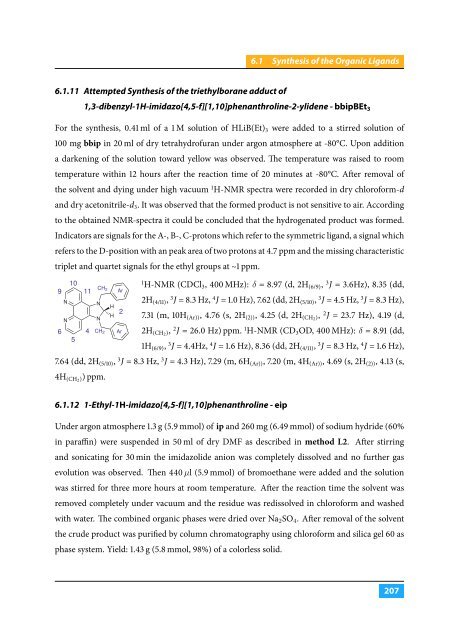
6.1.11 Attempted Synthesis of the triethylborane adduct of<br />
1,3-dibenzyl-1H-imidazo[4,5-f][1,10]phenanthroline-2-ylidene - bbipBEt 3<br />
For the synthesis, 0.41 ml of a 1 M solution of HLiB(Et) 3 were added to a stirred solution of<br />
100 mg bbip in 20 ml of dry tetrahydrofuran under argon atmosphere at -80°C. Upon addition<br />
a darkening of the solution toward yellow was observed. The temperature was raised to room<br />
temperature within 12 hours after the reaction time of 20 minutes at -80°C. After removal of<br />
the solvent and dying under high vacuum 1 H-NMR spectra were recorded in dry chloroform-d<br />
and dry acetonitrile-d 3 . It was observed that the formed product is not sensitive to air. According<br />
to the obtained NMR-spectra it could be concluded that the hydrogenated product was formed.<br />
Indicators are signals for the A-, B-, C-protons which refer to the symmetric ligand, a signal which<br />
refers to the D-position with an peak area of two protons at 4.7 ppm and the missing characteristic<br />
triplet and quartet signals for the ethyl groups at ∼1 ppm.<br />
9<br />
N<br />
N<br />
10<br />
11<br />
6 4<br />
5<br />
CH 2<br />
N<br />
N<br />
CH 2<br />
H<br />
H<br />
Ar<br />
2<br />
Ar<br />
1<br />
H-NMR (CDCl 3 , 400 MHz): δ = 8.97 (d, 2H (6/9) , 3 J = 3.6Hz), 8.35 (dd,<br />
2H (4/11) , 3 J = 8.3 Hz, 4 J = 1.0 Hz), 7.62 (dd, 2H (5/10) , 3 J = 4.5 Hz, 3 J = 8.3 Hz),<br />
7.31 (m, 10H (Ar)) , 4.76 (s, 2H (2)) , 4.25 (d, 2H (CH2 ), 2 J = 23.7 Hz), 4.19 (d,<br />
2H (CH2 ), 2 J = 26.0 Hz) ppm. 1 H-NMR (CD 3 OD, 400 MHz): δ = 8.91 (dd,<br />
1H (6/9) , 3 J = 4.4Hz, 4 J = 1.6 Hz), 8.36 (dd, 2H (4/11) , 3 J = 8.3 Hz, 4 J = 1.6 Hz),<br />
7.64 (dd, 2H (5/10) , 3 J = 8.3 Hz, 3 J = 4.3 Hz), 7.29 (m, 6H (Ar)) , 7.20 (m, 4H (Ar)) , 4.69 (s, 2H (2)) , 4.13 (s,<br />
4H (CH2 )) ppm.<br />
6.1.12 1-Ethyl-1H-imidazo[4,5-f][1,10]phenanthroline - eip<br />
Under argon atmosphere 1.3 g (5.9 mmol) of ip and 260 mg (6.49 mmol) of sodium hydride (60%<br />
in paraffin) were suspended in 50 ml of dry DMF as described in method L2. After stirring<br />
and sonicating for 30 min the imidazolide anion was completely dissolved and no further gas<br />
evolution was observed. Then 440 µl (5.9 mmol) of bromoethane were added and the solution<br />
was stirred for three more hours at room temperature. After the reaction time the solvent was<br />
removed completely under vacuum and the residue was redissolved in chloroform and washed<br />
with water. The combined organic phases were dried over Na 2 SO 4 . After removal of the solvent<br />
the crude product was purified by column chromatography using chloroform and silica gel 60 as<br />
phase system. Yield: 1.43 g (5.8 mmol, 98%) of a colorless solid.<br />
|207|