- Page 1 and 2: Development of Novel Catalysts for
- Page 3 and 4: Diese Arbeit entstand auf Anregung
- Page 5 and 6: Den Arbeisgruppen von Prof. Dr. U.
- Page 7 and 8: Contents 1 Introduction 1 1.1 Fossi
- Page 9 and 10: |Contents| 6 Experimental Section 1
- Page 12 and 13: |1 Introduction| 1 Introduction The
- Page 14 and 15: |1.1 Fossil Fuels and Nuclear Power
- Page 16 and 17: |1.2 Renewable Fuels| of natural ga
- Page 18 and 19: |1.3 Energy Transformation and Stor
- Page 20 and 21: |1.4 Solar Energy Conversion| A fra
- Page 22 and 23: |1.5 Photosynthesis| Figure 9: Mole
- Page 24 and 25: |1.6 Photocatalyzed Reactions| 2 NA
- Page 26 and 27: |1.6 Photocatalyzed Reactions| (1)
- Page 28 and 29: |1.7 Mimicking Photosynthesis| So t
- Page 30 and 31: |1.8 Formalisms of Photocatalytic S
- Page 32 and 33: |1.9 Multicomponent Systems from Fu
- Page 34 and 35: |1.9 Multicomponent Systems from Fu
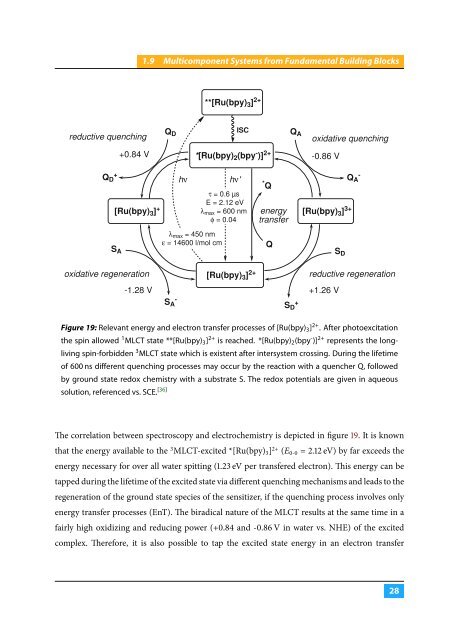
- Page 36 and 37: |1.9 Multicomponent Systems from Fu
- Page 40 and 41: |1.9 Multicomponent Systems from Fu
- Page 42 and 43: |1.9 Multicomponent Systems from Fu
- Page 44 and 45: |1.9 Multicomponent Systems from Fu
- Page 46 and 47: |1.9 Multicomponent Systems from Fu
- Page 48 and 49: |1.9 Multicomponent Systems from Fu
- Page 50 and 51: |1.9 Multicomponent Systems from Fu
- Page 52 and 53: |1.10 Intramolecular Photoredoxcata
- Page 54 and 55: |1.10 Intramolecular Photoredoxcata
- Page 56 and 57: |1.10 Intramolecular Photoredoxcata
- Page 58 and 59: |1.10 Intramolecular Photoredoxcata
- Page 60 and 61: |1.10 Intramolecular Photoredoxcata
- Page 62 and 63: |1.10 Intramolecular Photoredoxcata
- Page 64 and 65: |2 Scope of the Thesis| motifs with
- Page 66 and 67: |3.1 Brominated Phenanthrolines - A
- Page 68 and 69: |3.1 Brominated Phenanthrolines - A
- Page 70 and 71: |3.1 Brominated Phenanthrolines - A
- Page 72 and 73: |3.1 Brominated Phenanthrolines - A
- Page 74 and 75: |3.1 Brominated Phenanthrolines - A
- Page 76 and 77: |3.1 Brominated Phenanthrolines - A
- Page 78 and 79: |3.1 Brominated Phenanthrolines - A
- Page 80 and 81: |3.1 Brominated Phenanthrolines - A
- Page 82 and 83: |3.1 Brominated Phenanthrolines - A
- Page 84 and 85: |3.1 Brominated Phenanthrolines - A
- Page 86 and 87: |3.1 Brominated Phenanthrolines - A
- Page 88 and 89:
|3.1 Brominated Phenanthrolines - A
- Page 90 and 91:
|3.1 Brominated Phenanthrolines - A
- Page 92 and 93:
|3.1 Brominated Phenanthrolines - A
- Page 94 and 95:
|3.2 Bisphenanthroline: A Suitable
- Page 96 and 97:
|3.2 Bisphenanthroline: A Suitable
- Page 98 and 99:
|3.2 Bisphenanthroline: A Suitable
- Page 100 and 101:
|3.2 Bisphenanthroline: A Suitable
- Page 102 and 103:
|3.2 Bisphenanthroline: A Suitable
- Page 104 and 105:
f f f f f f |3.2 Bisphenanthroline:
- Page 106 and 107:
|3.2 Bisphenanthroline: A Suitable
- Page 108 and 109:
|3.2 Bisphenanthroline: A Suitable
- Page 110 and 111:
|3.2 Bisphenanthroline: A Suitable
- Page 112 and 113:
|3.2 Bisphenanthroline: A Suitable
- Page 114 and 115:
|3.2 Bisphenanthroline: A Suitable
- Page 116 and 117:
|3.2 Bisphenanthroline: A Suitable
- Page 118 and 119:
|3.2 Bisphenanthroline: A Suitable
- Page 120 and 121:
|3.2 Bisphenanthroline: A Suitable
- Page 122 and 123:
|3.3 NN-NHC-Ligand bbip: Toward Sec
- Page 124 and 125:
|3.3 NN-NHC-Ligand bbip: Toward Sec
- Page 126 and 127:
|3.3 NN-NHC-Ligand bbip: Toward Sec
- Page 128 and 129:
|3.3 NN-NHC-Ligand bbip: Toward Sec
- Page 130 and 131:
|3.3 NN-NHC-Ligand bbip: Toward Sec
- Page 132 and 133:
|3.3 NN-NHC-Ligand bbip: Toward Sec
- Page 134 and 135:
|3.3 NN-NHC-Ligand bbip: Toward Sec
- Page 136 and 137:
|3.3 NN-NHC-Ligand bbip: Toward Sec
- Page 138 and 139:
|3.3 NN-NHC-Ligand bbip: Toward Sec
- Page 140 and 141:
|3.3 NN-NHC-Ligand bbip: Toward Sec
- Page 142 and 143:
|3.3 NN-NHC-Ligand bbip: Toward Sec
- Page 144 and 145:
|3.3 NN-NHC-Ligand bbip: Toward Sec
- Page 146 and 147:
|3.3 NN-NHC-Ligand bbip: Toward Sec
- Page 148 and 149:
|3.3 NN-NHC-Ligand bbip: Toward Sec
- Page 150 and 151:
|3.3 NN-NHC-Ligand bbip: Toward Sec
- Page 152 and 153:
|3.3 NN-NHC-Ligand bbip: Toward Sec
- Page 154 and 155:
|3.3 NN-NHC-Ligand bbip: Toward Sec
- Page 156 and 157:
|3.3 NN-NHC-Ligand bbip: Toward Sec
- Page 158 and 159:
|3.3 NN-NHC-Ligand bbip: Toward Sec
- Page 160 and 161:
|3.3 NN-NHC-Ligand bbip: Toward Sec
- Page 162 and 163:
|3.3 NN-NHC-Ligand bbip: Toward Sec
- Page 164 and 165:
|3.3 NN-NHC-Ligand bbip: Toward Sec
- Page 166 and 167:
|3.3 NN-NHC-Ligand bbip: Toward Sec
- Page 168 and 169:
|3.3 NN-NHC-Ligand bbip: Toward Sec
- Page 170 and 171:
|3.4 Outlook, Exploratory Investiga
- Page 173 and 174:
|3.4 Outlook, Exploratory Investiga
- Page 175 and 176:
|3.4 Outlook, Exploratory Investiga
- Page 177 and 178:
|3.4 Outlook, Exploratory Investiga
- Page 179 and 180:
|3.4 Outlook, Exploratory Investiga
- Page 181 and 182:
|3.4 Outlook, Exploratory Investiga
- Page 183 and 184:
|3.4 Outlook, Exploratory Investiga
- Page 185 and 186:
|4 Summary| 4 Summary Against the b
- Page 187 and 188:
|4 Summary| The resulting complexes
- Page 189 and 190:
|4 Summary| 10 [TEA] + TEA visible
- Page 191 and 192:
|4 Summary| absorption between 430
- Page 193 and 194:
|4 Summary| additional NN- and NHC-
- Page 195 and 196:
|5 Zusammenfassung| Die Herstellung
- Page 197 and 198:
|5 Zusammenfassung| phenphen und Ru
- Page 199 and 200:
|5 Zusammenfassung| Fragment tragen
- Page 201 and 202:
|5 Zusammenfassung| [TEA] + TEA vis
- Page 203 and 204:
|5 Zusammenfassung| In einer abschl
- Page 205 and 206:
|6 Experimental Section| Spectroele
- Page 207 and 208:
|6 Experimental Section| 1.3648 was
- Page 209 and 210:
|6.1 Synthesis of the Organic Ligan
- Page 211 and 212:
|6.1 Synthesis of the Organic Ligan
- Page 213 and 214:
|6.1 Synthesis of the Organic Ligan
- Page 215 and 216:
|6.1 Synthesis of the Organic Ligan
- Page 217 and 218:
|6.1 Synthesis of the Organic Ligan
- Page 219 and 220:
|6.1 Synthesis of the Organic Ligan
- Page 221 and 222:
|6.1 Synthesis of the Organic Ligan
- Page 223 and 224:
|6.2 Synthesis of the Metal Complex
- Page 225 and 226:
|6.2 Synthesis of the Metal Complex
- Page 227 and 228:
|6.2 Synthesis of the Metal Complex
- Page 229 and 230:
|6.2 Synthesis of the Metal Complex
- Page 231 and 232:
|6.2 Synthesis of the Metal Complex
- Page 233 and 234:
|6.2 Synthesis of the Metal Complex
- Page 235 and 236:
|6.2 Synthesis of the Metal Complex
- Page 237 and 238:
|6.2 Synthesis of the Metal Complex
- Page 239 and 240:
|6.2 Synthesis of the Metal Complex
- Page 241 and 242:
|6.2 Synthesis of the Metal Complex
- Page 243 and 244:
|6.2 Synthesis of the Metal Complex
- Page 245 and 246:
|6.2 Synthesis of the Metal Complex
- Page 247 and 248:
|6.2 Synthesis of the Metal Complex
- Page 249 and 250:
|7 Appendix| 7 Appendix dd ddd DEI
- Page 251 and 252:
|7 Appendix| Ru(tpphz)Os [Ru(bpy) 2
- Page 253 and 254:
|References| [26] M. Chanon, M. Sch
- Page 255 and 256:
|References| [81] S. Tschierlei, M.
- Page 257 and 258:
|References| [138] L. Pazderski, J.
- Page 259 and 260:
|References| [194] C. Liu, J. Li, B
- Page 261:
Selbstständigkeitserklärung: Ich