Dissertation
Dissertation
Dissertation
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
|6.2 Synthesis of the Metal Complexes|<br />
6.2.3 Transmetallation of [Ag(NHC)X]-type complexes - method C3<br />
Under argon atmosphere one equivalent (e.g. 100 µmol) of [Ru(tbbpy) 2 (µ-bbip)AgCl]Cl 2 and one<br />
equivalent of the starting complex for the transmetallation reaction were dissolved in 20 ml of dry<br />
dichloromethane. After stirring for 2-16 hours at room temperature a colorless precipitate was<br />
formed. The solid was removed by filtration through oven dried celite after the reaction time. The<br />
celite was washed with dry dichloromethane or acetonitrile to obtain remaining product. Finally<br />
the solvent was removed completely under vacuum. The product was obtained as a red powder<br />
which decomposes under air. Yield: (∼95%).<br />
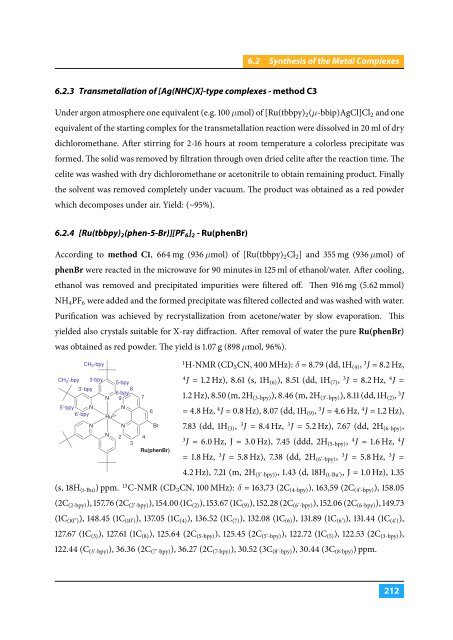
6.2.4 [Ru(tbbpy) 2 (phen-5-Br)][PF 6 ] 2 - Ru(phenBr)<br />
According to method C1, 664 mg (936 µmol) of [Ru(tbbpy) 2 Cl 2 ] and 355 mg (936 µmol) of<br />
phenBr were reacted in the microwave for 90 minutes in 125 ml of ethanol/water. After cooling,<br />
ethanol was removed and precipitated impurities were filtered off. Then 916 mg (5.62 mmol)<br />
NH 4 PF 6 were added and the formed precipitate was filtered collected and was washed with water.<br />
Purification was achieved by recrystallization from acetone/water by slow evaporation. This<br />
yielded also crystals suitable for X-ray diffraction. After removal of water the pure Ru(phenBr)<br />
was obtained as red powder. The yield is 1.07 g (898 µmol, 96%).<br />
CH 3 -bpy<br />
1<br />
H-NMR (CD 3 CN, 400 MHz): δ = 8.79 (dd, 1H (4) , 3 J = 8.2 Hz,<br />
CH 3 '-bpy 3-bpy<br />
5-bpy<br />
3'-bpy<br />
8<br />
6-bpy<br />
N 9 7<br />
5'-bpy N<br />
N<br />
6<br />
6'-bpy<br />
Ru 2+<br />
N<br />
N<br />
Br<br />
N 2 4<br />
3<br />
Ru(phenBr)<br />
4<br />
J = 1.2 Hz), 8.61 (s, 1H (6) ), 8.51 (dd, 1H (7) , 3 J = 8.2 Hz, 4 J =<br />
1.2 Hz), 8.50 (m, 2H (3-bpy) ), 8.46 (m, 2H (3’-bpy) ), 8.11 (dd, 1H (2) , 3 J<br />
= 4.8 Hz, 4 J = 0.8 Hz), 8.07 (dd, 1H (9) , 3 J = 4.6 Hz, 4 J = 1.2 Hz),<br />
7.83 (dd, 1H (3) , 3 J = 8.4 Hz, 3 J = 5.2 Hz), 7.67 (dd, 2H (6-bpy) ,<br />
3<br />
J = 6.0 Hz, J = 3.0 Hz), 7.45 (ddd, 2H (5-bpy) , 4 J = 1.6 Hz, 4 J<br />
= 1.8 Hz, 3 J = 5.8 Hz), 7.38 (dd, 2H (6’-bpy) , 3 J = 5.8 Hz, 3 J =<br />
4.2 Hz), 7.21 (m, 2H (5’-bpy)) , 1.43 (d, 18H (t-Bu’) , J = 1.0 Hz), 1.35<br />
(s, 18H (t-Bu) ) ppm. 13 C-NMR (CD 3 CN, 100 MHz): δ = 163,73 (2C (4-bpy) ), 163,59 (2C (4’-bpy) ), 158.05<br />
(2C (2-bpy) ), 157.76 (2C (2’-bpy) ), 154.00 (1C (2) ), 153.67 (1C (9) ), 152.28 (2C (6’-bpy) ), 152.06 (2C (6-bpy) ), 149.73<br />
(1C (10”) ), 148.45 (1C (10’) ), 137.05 (1C (4) ), 136.52 (1C (7) ), 132.08 (1C (6) ), 131.89 (1C (6’) ), 131.44 (1C (4’) ),<br />
127.67 (1C (3) ), 127.61 (1C (8) ), 125.64 (2C (5-bpy) ), 125.45 (2C (5’-bpy) ), 122.72 (1C (5) ), 122.53 (2C (3-bpy) ),<br />
122.44 (C (3’-bpy) ), 36.36 (2C (7’-bpy) ), 36.27 (2C (7-bpy) ), 30.52 (3C (8’-bpy) ), 30.44 (3C (8-bpy) ) ppm.<br />
|212|