Dissertation
Dissertation
Dissertation
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
|6.1 Synthesis of the Organic Ligands|<br />
was collected after cooling and washed with ether to give 163 mg (200 µmol, 50%) of the desired<br />
product as white off-white solid. Recrystallization from D 2 O yielded suitable crystals for X-ray<br />
diffraction.<br />
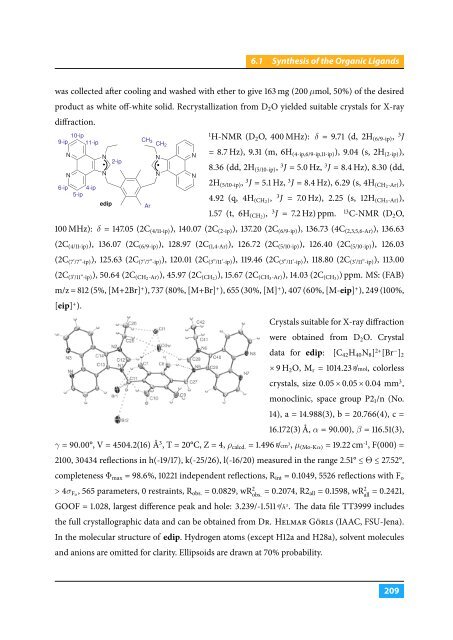
9-ip<br />
6-ip<br />
N<br />
N<br />
10-ip<br />
11-ip<br />
4-ip<br />
5-ip<br />
N<br />
N<br />
edip<br />
2-ip<br />
CH 3<br />
CH 2<br />
Ar<br />
N<br />
N<br />
N<br />
N<br />
1<br />
H-NMR (D 2 O, 400 MHz): δ = 9.71 (d, 2H (6/9-ip) , 3 J<br />
= 8.7 Hz), 9.31 (m, 6H (4-ip,6/9-ip,11-ip) ), 9.04 (s, 2H (2-ip) ),<br />
8.36 (dd, 2H (5/10-ip) , 3 J = 5.0 Hz, 3 J = 8.4 Hz), 8.30 (dd,<br />
2H (5/10-ip) , 3 J = 5.1 Hz, 3 J = 8.4 Hz), 6.29 (s, 4H (CH2 -Ar)),<br />
4.92 (q, 4H (CH2 ), 3<br />
J = 7.0 Hz), 2.25 (s, 12H (CH3 -Ar)),<br />
1.57 (t, 6H (CH2 ), 3<br />
J = 7.2 Hz) ppm.<br />
13<br />
C-NMR (D 2 O,<br />
100 MHz): δ = 147.05 (2C (4/11-ip) ), 140.07 (2C (2-ip) ), 137.20 (2C (6/9-ip) ), 136.73 (4C (2,3,5,6-Ar) ), 136.63<br />
(2C (4/11-ip) ), 136.07 (2C (6/9-ip) ), 128.97 (2C (1,4-Ar) ), 126.72 (2C (5/10-ip) ), 126.40 (2C (5/10-ip) ), 126.03<br />
(2C (7’/7”-ip) ), 125.63 (2C (7’/7”-ip) ), 120.01 (2C (3”/11’-ip) ), 119.46 (2C (3”/11’-ip) ), 118.80 (2C (3’/11”-ip) ), 113.00<br />
(2C (3’/11”-ip) ), 50.64 (2C (CH2 -Ar)), 45.97 (2C (CH2 )), 15.67 (2C (CH3 -Ar)), 14.03 (2C (CH3 )) ppm. MS: (FAB)<br />
m/z = 812 (5%, [M+2Br] + ), 737 (80%, [M+Br] + ), 655 (30%, [M] + ), 407 (60%, [M-eip] + ), 249 (100%,<br />
[eip] + ).<br />
Crystals suitable for X-ray diffraction<br />
were obtained from D 2 O. Crystal<br />
data for edip: [C 42 H 40 N 8 ] 2+ [Br − ] 2<br />
× 9 H 2 O, M r = 1014.23 g /mol, colorless<br />
crystals, size 0.05 × 0.05 × 0.04 mm 3 ,<br />
monoclinic, space group P2 1 /n (No.<br />
14), a = 14.988(3), b = 20.766(4), c =<br />
16.172(3) Å, α = 90.00), β = 116.51(3),<br />
γ = 90.00°, V = 4504.2(16) Å 3 , T = 20°C, Z = 4, ρ calcd. = 1.496 g /cm 3 , µ (Mo-Kα) = 19.22 cm -1 , F(000) =<br />
2100, 30434 reflections in h(-19/17), k(-25/26), l(-16/20) measured in the range 2.51° ≤ Θ ≤ 27.52°,<br />
completeness Φ max = 98.6%, 10221 independent reflections, R int = 0.1049, 5526 reflections with F o<br />
> 4σ Fo , 565 parameters, 0 restraints, R obs. = 0.0829, wR 2 obs. = 0.2074, R2 all = 0.1598, wR 2 all = 0.2421,<br />
GOOF = 1.028, largest difference peak and hole: 3.239/-1.511 e /Å 3 . The data file TT3999 includes<br />
the full crystallographic data and can be obtained from Dr. Helmar Görls (IAAC, FSU-Jena).<br />
In the molecular structure of edip. Hydrogen atoms (except H12a and H28a), solvent molecules<br />
and anions are omitted for clarity. Ellipsoids are drawn at 70% probability.<br />
|209|