Dissertation
Dissertation
Dissertation
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
|6.2 Synthesis of the Metal Complexes|<br />
137.34 (2C (2,3,5,6-Durol) ), 131.49 (2C (4-ip) ), 130.74 (2C (11-ip) ), 129.38 (2C (1,4-Durol) ), 127.50 (2C (3’) ), 127.14<br />
(2C (5) ), 126.96 (2C (11”) ), 126.74 (2C (10) ), 124.82 (4C (5-bpy) ), 124.58 (4C (5’-bpy) ), 121.75 (8C (6/6’-bpy) ),<br />
121.66 (2C (3”-ip) ), 121.03 (2C (11’-ip) ), 51.00 (2C (CH2 -Ar)), 46.42 (2C (CH2 -Al)), 35.48 (2C (C-tBu) ), 35.37<br />
(2C (C-tBu) ), 29.58 (12C (CH3 - t Bu)), 29.49 (12C (CH3 - t Bu)), 16.19 (4C (CH3 -Ar)), 14.33 (2C (CH3 -Al)) ppm. MS<br />
(ESI): m/z = 1110.2 (10%, [M - 2 PF 6 ] 2+ ) 1031.1 (100%, [Ru(eip)+ PF 6 ] + ).<br />
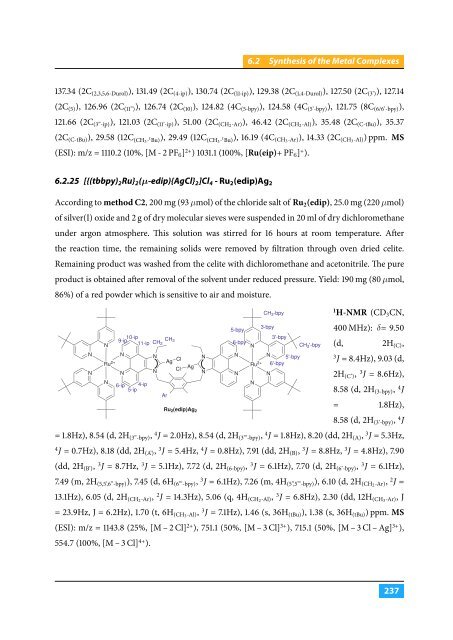
6.2.25 [{(tbbpy) 2 Ru} 2 (µ-edip){AgCl} 2 ]Cl 4 - Ru 2 (edip)Ag 2<br />
According to method C2, 200 mg (93 µmol) of the chloride salt of Ru 2 (edip), 25.0 mg (220 µmol)<br />
of silver(I) oxide and 2 g of dry molecular sieves were suspended in 20 ml of dry dichloromethane<br />
under argon atmosphere. This solution was stirred for 16 hours at room temperature. After<br />
the reaction time, the remaining solids were removed by filtration through oven dried celite.<br />
Remaining product was washed from the celite with dichloromethane and acetonitrile. The pure<br />
product is obtained after removal of the solvent under reduced pressure. Yield: 190 mg (80 µmol,<br />
86%) of a red powder which is sensitive to air and moisture.<br />
CH 3 -bpy<br />
1<br />
H-NMR (CD 3 CN,<br />
N<br />
N<br />
N<br />
9-ip 10-ip CH<br />
11-ip CH 3 2<br />
N<br />
N<br />
N<br />
Ru 2+ Ag<br />
Cl<br />
N<br />
N<br />
Cl<br />
Ag<br />
N<br />
N<br />
6-ip 4-ip<br />
5-ip<br />
Ar<br />
Ru 2 (edip)Ag 2<br />
5-bpy<br />
6-bpy<br />
N<br />
N<br />
N<br />
Ru 2+<br />
N<br />
3-bpy<br />
3'-bpy<br />
N 5'-bpy<br />
6'-bpy<br />
N<br />
CH 3 '-bpy<br />
400 MHz): δ= 9.50<br />
(d, 2H (C) ,<br />
3<br />
J = 8.4Hz), 9.03 (d,<br />
2H (C’) , 3 J = 8.6Hz),<br />
8.58 (d, 2H (3-bpy) , 4 J<br />
= 1.8Hz),<br />
8.58 (d, 2H (3’-bpy) , 4 J<br />
= 1.8Hz), 8.54 (d, 2H (3”-bpy) , 4 J = 2.0Hz), 8.54 (d, 2H (3”’-bpy) , 4 J = 1.8Hz), 8.20 (dd, 2H (A) , 3 J = 5.3Hz,<br />
4<br />
J = 0.7Hz), 8.18 (dd, 2H (A’) , 3 J = 5.4Hz, 4 J = 0.8Hz), 7.91 (dd, 2H (B) , 3 J = 8.8Hz, 3 J = 4.8Hz), 7.90<br />
(dd, 2H (B’) , 3 J = 8.7Hz, 3 J = 5.1Hz), 7.72 (d, 2H (6-bpy) , 3 J = 6.1Hz), 7.70 (d, 2H (6’-bpy) , 3 J = 6.1Hz),<br />
7.49 (m, 2H (5,5’,6”-bpy) ), 7.45 (d, 6H (6”’-bpy) , 3 J = 6.1Hz), 7.26 (m, 4H (5”,5”’-bpy) ), 6.10 (d, 2H (CH2 -Ar), 2 J =<br />
13.1Hz), 6.05 (d, 2H (CH2 -Ar), 2 J = 14.3Hz), 5.06 (q, 4H (CH2 -Al), 3 J = 6.8Hz), 2.30 (dd, 12H (CH3 -Ar), J<br />
= 23.9Hz, J = 6.2Hz), 1.70 (t, 6H (CH3 -Al), 3 J = 7.1Hz), 1.46 (s, 36H (tBu) ), 1.38 (s, 36H (tBu) ) ppm. MS<br />
(ESI): m/z = 1143.8 (25%, [M – 2 Cl] 2+ ), 751.1 (50%, [M – 3 Cl] 3+ ), 715.1 (50%, [M – 3 Cl – Ag] 3+ ),<br />
554.7 (100%, [M – 3 Cl] 4+ ).<br />
|237|