Dissertation
Dissertation
Dissertation
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
|3.4 Outlook, Exploratory Investigations and Perspectives|<br />
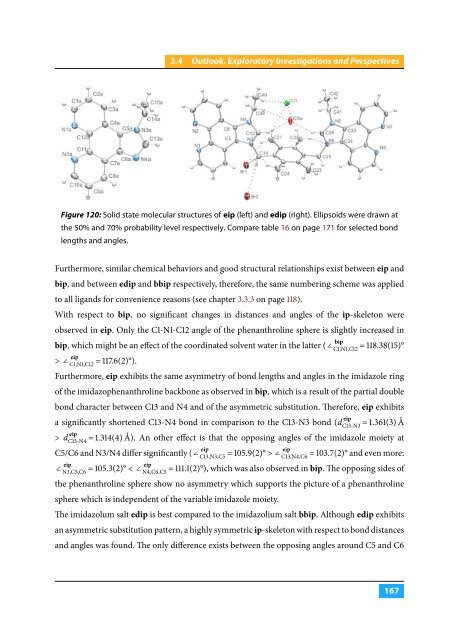
Figure 120: Solid state molecular structures of eip (left) and edip (right). Ellipsoids were drawn at<br />
the 50% and 70% probability level respectively. Compare table 16 on page 171 for selected bond<br />
lengths and angles.<br />
Furthermore, similar chemical behaviors and good structural relationships exist between eip and<br />
bip, and between edip and bbip respectively, therefore, the same numbering scheme was applied<br />
to all ligands for convenience reasons (see chapter 3.3.3 on page 118).<br />
With respect to bip, no significant changes in distances and angles of the ip-skeleton were<br />
observed in eip. Only the C1-N1-C12 angle of the phenanthroline sphere is slightly increased in<br />
bip, which might be an effect of the coordinated solvent water in the latter (∠ bip<br />
C1,N1,C12 = 118.38(15)°<br />
> ∠ eip<br />
C1,N1,C12 = 117.6(2)°).<br />
Furthermore, eip exhibits the same asymmetry of bond lengths and angles in the imidazole ring<br />
of the imidazophenanthroline backbone as observed in bip, which is a result of the partial double<br />
bond character between C13 and N4 and of the asymmetric substitution. Therefore, eip exhibits<br />
a significantly shortened C13-N4 bond in comparison to the C13-N3 bond (d eip<br />
C13-N3 = 1.361(3) Å<br />
> d eip<br />
C13-N4<br />
= 1.314(4) Å). An other effect is that the opposing angles of the imidazole moiety at<br />
C5/C6 and N3/N4 differ significantly (∠ eip<br />
eip<br />
C13,N3,C5<br />
= 105.9(2)° > ∠C13,N4,C6 = 103.7(2)° and even more:<br />
∠ eip<br />
eip<br />
N3,C5,C6<br />
= 105.3(2)° < ∠N4,C6,C5 = 111.1(2)°), which was also observed in bip. The opposing sides of<br />
the phenanthroline sphere show no asymmetry which supports the picture of a phenanthroline<br />
sphere which is independent of the variable imidazole moiety.<br />
The imidazolum salt edip is best compared to the imidazolium salt bbip. Although edip exhibits<br />
an asymmetric substitution pattern, a highly symmetric ip-skeleton with respect to bond distances<br />
and angles was found. The only difference exists between the opposing angles around C5 and C6<br />
|167|