Boreskov Institute of Catalysis SB RAS, Novosibirsk, Russia
Boreskov Institute of Catalysis SB RAS, Novosibirsk, Russia
Boreskov Institute of Catalysis SB RAS, Novosibirsk, Russia
- No tags were found...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
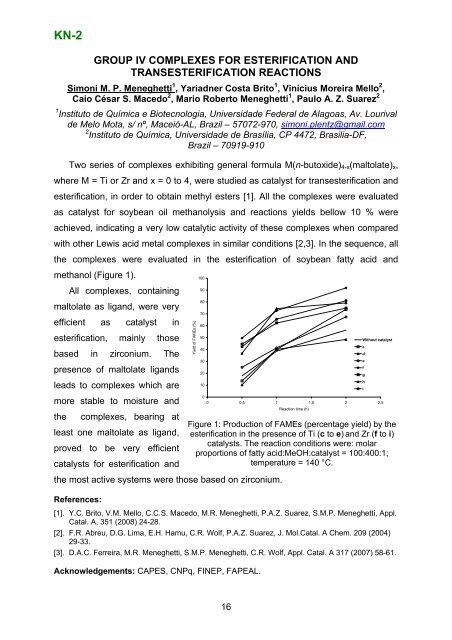
KN-2GROUP IV COMPLEXES FOR ESTERIFICATION ANDTRANSESTERIFICATION REACTIONSSimoni M. P. Meneghetti 1 , Yariadner Costa Brito 1 , Vinícius Moreira Mello 2 ,Caio César S. Macedo 2 , Mario Roberto Meneghetti 1 , Paulo A. Z. Suarez 21 Instituto de Química e Biotecnologia, Universidade Federal de Alagoas, Av. Lourivalde Melo Mota, s/ nº, Maceió-AL, Brazil – 57072-970, simoni.plentz@gmail.com2 Instituto de Química, Universidade de Brasília, CP 4472, Brasilia-DF,Brazil – 70919-910Two series <strong>of</strong> complexes exhibiting general formula M(n-butoxide) 4-x (maltolate) x ,where M = Ti or Zr and x = 0 to 4, were studied as catalyst for transesterification andesterification, in order to obtain methyl esters [1]. All the complexes were evaluatedas catalyst for soybean oil methanolysis and reactions yields bellow 10 % wereachieved, indicating a very low catalytic activity <strong>of</strong> these complexes when comparedwith other Lewis acid metal complexes in similar conditions [2,3]. In the sequence, allthe complexes were evaluated in the esterification <strong>of</strong> soybean fatty acid andmethanol (Figure 1).10090All complexes, containing80maltolate as ligand, were very70efficient as catalyst in6050esterification, mainly thoseWithout catalystc40based in zirconium. Thed30efpresence <strong>of</strong> maltolate ligands20ghleads to complexes which are10i0more stable to moisture and0 0,5 1 1,5 2 2,5Reaction time (h)the complexes, bearing atFigure 1: Production <strong>of</strong> FAMEs (percentage yield) by theleast one maltolate as ligand, esterification in the presence <strong>of</strong> Ti (c to e) and Zr (f to i)catalysts. The reaction conditions were: molarproved to be very efficientproportions <strong>of</strong> fatty acid:MeOH:catalyst = 100:400:1;catalysts for esterification andtemperature = 140 °C.the most active systems were those based on zirconium.References:Yi eld <strong>of</strong> FAM E s (%)[1]. Y.C. Brito, V.M. Mello, C.C.S. Macedo, M.R. Meneghetti, P.A.Z. Suarez, S.M.P. Meneghetti, Appl.Catal. A, 351 (2008) 24-28.[2]. F.R. Abreu, D.G. Lima, E.H. Hamu, C.R. Wolf, P.A.Z. Suarez, J. Mol.Catal. A Chem. 209 (2004)29-33.[3]. D.A.C. Ferreira, M.R. Meneghetti, S.M.P. Meneghetti, C.R. Wolf, Appl. Catal. A 317 (2007) 58-61.Acknowledgements: CAPES, CNPq, FINEP, FAPEAL.16