Boreskov Institute of Catalysis SB RAS, Novosibirsk, Russia
Boreskov Institute of Catalysis SB RAS, Novosibirsk, Russia
Boreskov Institute of Catalysis SB RAS, Novosibirsk, Russia
- No tags were found...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
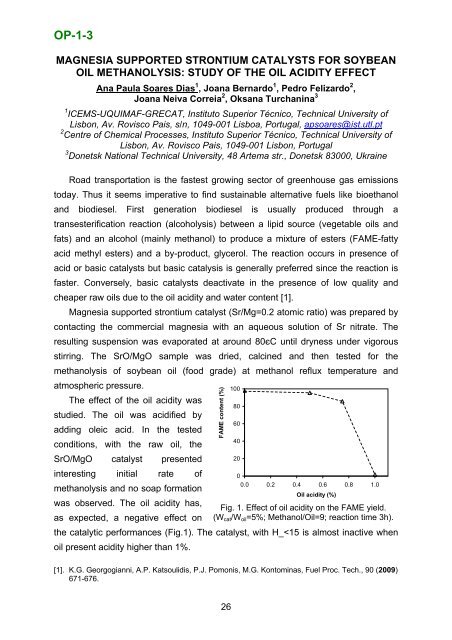
OP-1-3MAGNESIA SUPPORTED STRONTIUM CATALYSTS FOR SOYBEANOIL METHANOLYSIS: STUDY OF THE OIL ACIDITY EFFECTAna Paula Soares Dias 1 , Joana Bernardo 1 , Pedro Felizardo 2 ,Joana Neiva Correia 2 , Oksana Turchanina 31 ICEMS-UQUIMAF-GRECAT, Instituto Superior Técnico, Technical University <strong>of</strong>Lisbon, Av. Rovisco Pais, s/n, 1049-001 Lisboa, Portugal, apsoares@ist.utl.pt2 Centre <strong>of</strong> Chemical Processes, Instituto Superior Técnico, Technical University <strong>of</strong>Lisbon, Av. Rovisco Pais, 1049-001 Lisbon, Portugal3 Donetsk National Technical University, 48 Artema str., Donetsk 83000, UkraineRoad transportation is the fastest growing sector <strong>of</strong> greenhouse gas emissionstoday. Thus it seems imperative to find sustainable alternative fuels like bioethanoland biodiesel. First generation biodiesel is usually produced through atransesterification reaction (alcoholysis) between a lipid source (vegetable oils andfats) and an alcohol (mainly methanol) to produce a mixture <strong>of</strong> esters (FAME-fattyacid methyl esters) and a by-product, glycerol. The reaction occurs in presence <strong>of</strong>acid or basic catalysts but basic catalysis is generally preferred since the reaction isfaster. Conversely, basic catalysts deactivate in the presence <strong>of</strong> low quality andcheaper raw oils due to the oil acidity and water content [1].Magnesia supported strontium catalyst (Sr/Mg=0.2 atomic ratio) was prepared bycontacting the commercial magnesia with an aqueous solution <strong>of</strong> Sr nitrate. Theresulting suspension was evaporated at around 80єC until dryness under vigorousstirring. The SrO/MgO sample was dried, calcined and then tested for themethanolysis <strong>of</strong> soybean oil (food grade) at methanol reflux temperature andatmospheric pressure.100The effect <strong>of</strong> the oil acidity was80studied. The oil was acidified byadding oleic acid. In the testedconditions, with the raw oil, the6040SrO/MgO catalyst presented20interesting initial rate <strong>of</strong>0methanolysis and no soap formation0.0 0.2 0.4 0.6 0.8 1.0Oil acidity (%)was observed. The oil acidity has,as expected, a negative effect onFig. 1. Effect <strong>of</strong> oil acidity on the FAME yield.(W cat /W oil =5%; Methanol/Oil=9; reaction time 3h).the catalytic performances (Fig.1). The catalyst, with H_