Boreskov Institute of Catalysis SB RAS, Novosibirsk, Russia
Boreskov Institute of Catalysis SB RAS, Novosibirsk, Russia
Boreskov Institute of Catalysis SB RAS, Novosibirsk, Russia
- No tags were found...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
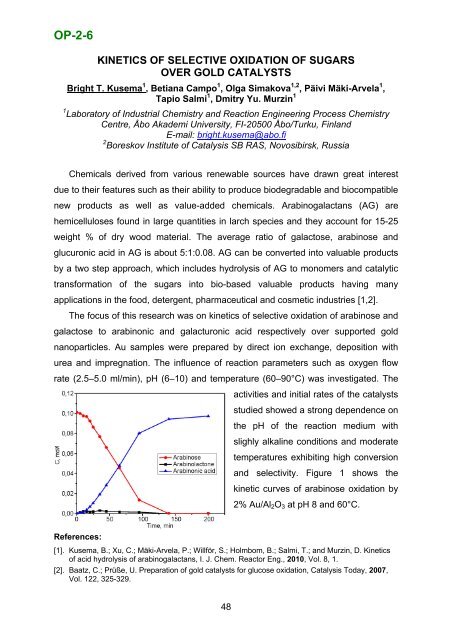
OP-2-6KINETICS OF SELECTIVE OXIDATION OF SUGARSOVER GOLD CATALYST<strong>SB</strong>right T. Kusema 1 , Betiana Campo 1 , Olga Simakova 1,2 , Päivi Mäki-Arvela 1 ,Tapio Salmi 1 , Dmitry Yu. Murzin 11 Laboratory <strong>of</strong> Industrial Chemistry and Reaction Engineering Process ChemistryCentre, Åbo Akademi University, FI-20500 Åbo/Turku, FinlandE-mail: bright.kusema@abo.fi2 <strong>Boreskov</strong> <strong>Institute</strong> <strong>of</strong> <strong>Catalysis</strong> <strong>SB</strong> <strong>RAS</strong>, <strong>Novosibirsk</strong>, <strong>Russia</strong>Chemicals derived from various renewable sources have drawn great interestdue to their features such as their ability to produce biodegradable and biocompatiblenew products as well as value-added chemicals. Arabinogalactans (AG) arehemicelluloses found in large quantities in larch species and they account for 15-25weight % <strong>of</strong> dry wood material. The average ratio <strong>of</strong> galactose, arabinose andglucuronic acid in AG is about 5:1:0.08. AG can be converted into valuable productsby a two step approach, which includes hydrolysis <strong>of</strong> AG to monomers and catalytictransformation <strong>of</strong> the sugars into bio-based valuable products having manyapplications in the food, detergent, pharmaceutical and cosmetic industries [1,2].The focus <strong>of</strong> this research was on kinetics <strong>of</strong> selective oxidation <strong>of</strong> arabinose andgalactose to arabinonic and galacturonic acid respectively over supported goldnanoparticles. Au samples were prepared by direct ion exchange, deposition withurea and impregnation. The influence <strong>of</strong> reaction parameters such as oxygen flowrate (2.5–5.0 ml/min), pH (6–10) and temperature (60–90°C) was investigated. Theactivities and initial rates <strong>of</strong> the catalystsstudied showed a strong dependence onthe pH <strong>of</strong> the reaction medium withslighly alkaline conditions and moderatetemperatures exhibiting high conversionand selectivity. Figure 1 shows thekinetic curves <strong>of</strong> arabinose oxidation by2% Au/Al 2 O 3 at pH 8 and 60°C.References:[1]. Kusema, B.; Xu, C.; Mäki-Arvela, P.; Willför, S.; Holmbom, B.; Salmi, T.; and Murzin, D. Kinetics<strong>of</strong> acid hydrolysis <strong>of</strong> arabinogalactans, I. J. Chem. Reactor Eng., 2010, Vol. 8, 1.[2]. Baatz, C.; Prüße, U. Preparation <strong>of</strong> gold catalysts for glucose oxidation, <strong>Catalysis</strong> Today, 2007,Vol. 122, 325-329.48