Boreskov Institute of Catalysis SB RAS, Novosibirsk, Russia
Boreskov Institute of Catalysis SB RAS, Novosibirsk, Russia
Boreskov Institute of Catalysis SB RAS, Novosibirsk, Russia
- No tags were found...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
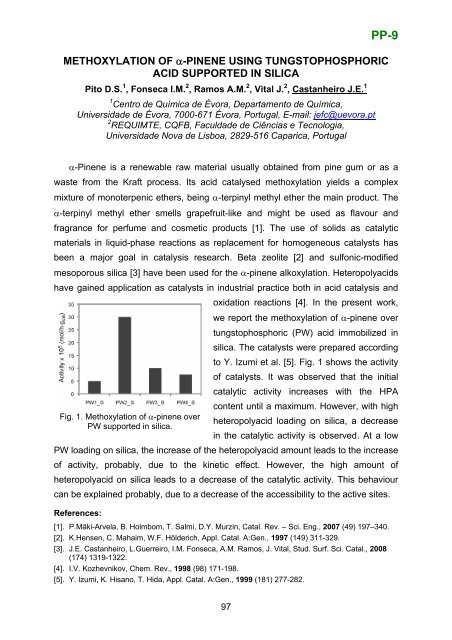
PP-9METHOXYLATION OF α-PINENE USING TUNGSTOPHOSPHORICACID SUPPORTED IN SILICAPito D.S. 1 , Fonseca I.M. 2 , Ramos A.M. 2 , Vital J. 2 , Castanheiro J.E. 11 Centro de Química de Évora, Departamento de Química,Universidade de Évora, 7000-671 Évora, Portugal, E-mail: jefc@uevora.pt2 REQUIMTE, CQFB, Faculdade de Ciências e Tecnologia,Universidade Nova de Lisboa, 2829-516 Caparica, Portugalα-Pinene is a renewable raw material usually obtained from pine gum or as awaste from the Kraft process. Its acid catalysed methoxylation yields a complexmixture <strong>of</strong> monoterpenic ethers, being α-terpinyl methyl ether the main product. Theα-terpinyl methyl ether smells grapefruit-like and might be used as flavour andfragrance for perfume and cosmetic products [1]. The use <strong>of</strong> solids as catalyticmaterials in liquid-phase reactions as replacement for homogeneous catalysts hasbeen a major goal in catalysis research. Beta zeolite [2] and sulfonic-modifiedmesoporous silica [3] have been used for the α-pinene alkoxylation. Heteropolyacidshave gained application as catalysts in industrial practice both in acid catalysis andoxidation reactions [4]. In the present work,we report the methoxylation <strong>of</strong> α-pinene overtungstophosphoric (PW) acid immobilized insilica. The catalysts were prepared accordingto Y. Izumi et al. [5]. Fig. 1 shows the activity<strong>of</strong> catalysts. It was observed that the initialcatalytic activity increases with the HPAcontent until a maximum. However, with highFig. 1. Methoxylation <strong>of</strong> α-pinene overheteropolyacid loading on silica, a decreasePW supported in silica.in the catalytic activity is observed. At a lowPW loading on silica, the increase <strong>of</strong> the heteropolyacid amount leads to the increase<strong>of</strong> activity, probably, due to the kinetic effect. However, the high amount <strong>of</strong>heteropolyacid on silica leads to a decrease <strong>of</strong> the catalytic activity. This behaviourcan be explained probably, due to a decrease <strong>of</strong> the accessibility to the active sites.References:[1]. P.Mäki-Arvela, B. Holmbom, T. Salmi, D.Y. Murzin, Catal. Rev. – Sci. Eng., 2007 (49) 197–340.[2]. K.Hensen, C. Mahaim, W.F. Hölderich, Appl. Catal. A:Gen., 1997 (149) 311-329.[3]. J.E. Castanheiro, L.Guerreiro, I.M. Fonseca, A.M. Ramos, J. Vital, Stud. Surf. Sci. Catal., 2008(174) 1319-1322.[4]. I.V. Kozhevnikov, Chem. Rev., 1998 (98) 171-198.[5]. Y. Izumi, K. Hisano, T. Hida, Appl. Catal. A:Gen., 1999 (181) 277-282.97