Boreskov Institute of Catalysis SB RAS, Novosibirsk, Russia
Boreskov Institute of Catalysis SB RAS, Novosibirsk, Russia
Boreskov Institute of Catalysis SB RAS, Novosibirsk, Russia
- No tags were found...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
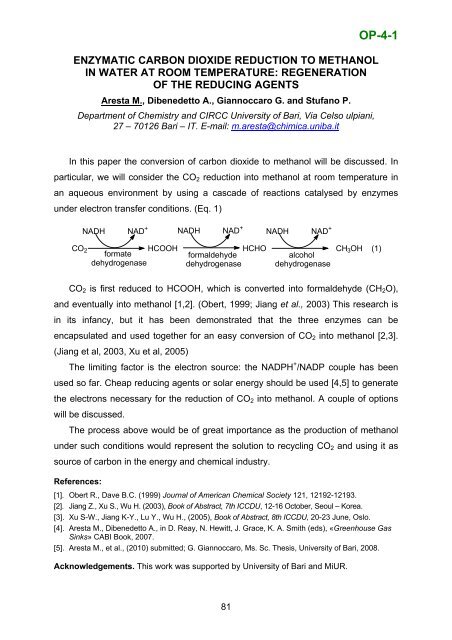
OP-4-1ENZYMATIC CARBON DIOXIDE REDUCTION TO METHANOLIN WATER AT ROOM TEMPERATURE: REGENERATIONOF THE REDUCING AGENTSAresta M., Dibenedetto A., Giannoccaro G. and Stufano P.Department <strong>of</strong> Chemistry and CIRCC University <strong>of</strong> Bari, Via Celso ulpiani,27 – 70126 Bari – IT. E-mail: m.aresta@chimica.uniba.itIn this paper the conversion <strong>of</strong> carbon dioxide to methanol will be discussed. Inparticular, we will consider the CO 2 reduction into methanol at room temperature inan aqueous environment by using a cascade <strong>of</strong> reactions catalysed by enzymesunder electron transfer conditions. (Eq. 1)NADH NAD + NADH NAD +NADH NAD +CO 2 HCOOH HCHO CH 3 OH (1)formateformaldehydealcoholdehydrogenase dehydrogenase dehydrogenaseCO 2 is first reduced to HCOOH, which is converted into formaldehyde (CH 2 O),and eventually into methanol [1,2]. (Obert, 1999; Jiang et al., 2003) This research isin its infancy, but it has been demonstrated that the three enzymes can beencapsulated and used together for an easy conversion <strong>of</strong> CO 2 into methanol [2,3].(Jiang et al, 2003, Xu et al, 2005)The limiting factor is the electron source: the NADPH + /NADP couple has beenused so far. Cheap reducing agents or solar energy should be used [4,5] to generatethe electrons necessary for the reduction <strong>of</strong> CO 2 into methanol. A couple <strong>of</strong> optionswill be discussed.The process above would be <strong>of</strong> great importance as the production <strong>of</strong> methanolunder such conditions would represent the solution to recycling CO 2 and using it assource <strong>of</strong> carbon in the energy and chemical industry.References:[1]. Obert R., Dave B.C. (1999) Journal <strong>of</strong> American Chemical Society 121, 12192-12193.[2]. Jiang Z., Xu S., Wu H. (2003), Book <strong>of</strong> Abstract, 7th ICCDU, 12-16 October, Seoul – Korea.[3]. Xu S-W., Jiang K-Y., Lu Y., Wu H., (2005), Book <strong>of</strong> Abstract, 8th ICCDU, 20-23 June, Oslo.[4]. Aresta M., Dibenedetto A., in D. Reay, N. Hewitt, J. Grace, K. A. Smith (eds), «Greenhouse GasSinks» CABI Book, 2007.[5]. Aresta M., et al., (2010) submitted; G. Giannoccaro, Ms. Sc. Thesis, University <strong>of</strong> Bari, 2008.Acknowledgements. This work was supported by University <strong>of</strong> Bari and MiUR.81