IJUP08 - Universidade do Porto
IJUP08 - Universidade do Porto
IJUP08 - Universidade do Porto
- TAGS
- universidade
- porto
- ijup.up.pt
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
DFT STUDY ON THE ABILITY OF CALIX[2]FURANO[2]PYRROLE<br />
TO FORM HOST-GUEST COMPLEXES WITH DIFFERENT IONS<br />
C.A. Teixeira <strong>do</strong>s Santos, and A.L. Magalhães<br />
Department of Chemistry, Faculty of Science, University of <strong>Porto</strong>, Portugal.<br />
The applications of calixarene derivatives as new receptors have attracted considerable<br />
interest in the area of host-guest chemistry[1]. The presence of sulfur or nitrogen atoms in<br />
the ring linkages or upper rim seems to greatly enhance the complexation abilities toward<br />
transition metal ions, when compared to the classical calixarene systems. Applications of<br />
these molecules as new receptors, building blocks and /or molecular platforms have<br />
attracted considerable interest in a wide range of areas such as host-guest chemistry,<br />
separation chemistry, environment protection, selective ion transport and sensors [1].<br />
Recently, DFT methods have been applied successfully in the study of the structural<br />
features and conformational equilibrium of sulfur bridged derivative compounds[2].<br />
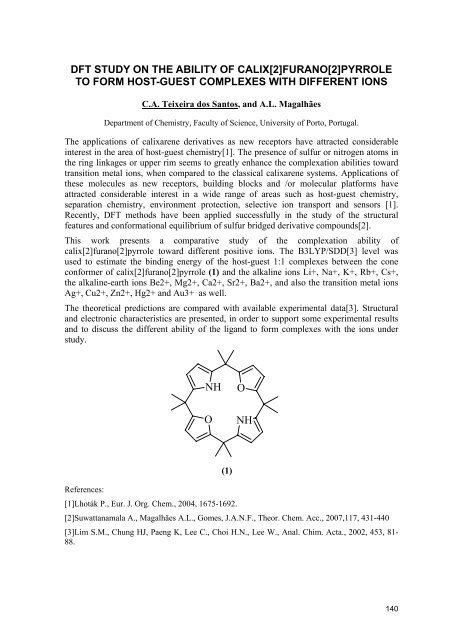
This work presents a comparative study of the complexation ability of<br />
calix[2]furano[2]pyrrole toward different positive ions. The B3LYP/SDD[3] level was<br />
used to estimate the binding energy of the host-guest 1:1 complexes between the cone<br />
conformer of calix[2]furano[2]pyrrole (1) and the alkaline ions Li+, Na+, K+, Rb+, Cs+,<br />
the alkaline-earth ions Be2+, Mg2+, Ca2+, Sr2+, Ba2+, and also the transition metal ions<br />
Ag+, Cu2+, Zn2+, Hg2+ and Au3+ as well.<br />
The theoretical predictions are compared with available experimental data[3]. Structural<br />
and electronic characteristics are presented, in order to support some experimental results<br />
and to discuss the different ability of the ligand to form complexes with the ions under<br />
study.<br />
NH<br />
O<br />
(1)<br />
References:<br />
[1]Lhoták P., Eur. J. Org. Chem., 2004, 1675-1692.<br />
[2]Suwattanamala A., Magalhães A.L., Gomes, J.A.N.F., Theor. Chem. Acc., 2007,117, 431-440<br />
[3]Lim S.M., Chung HJ, Paeng K, Lee C., Choi H.N., Lee W., Anal. Chim. Acta., 2002, 453, 81-<br />
88.<br />
O<br />
NH<br />
140