IJUP08 - Universidade do Porto
IJUP08 - Universidade do Porto
IJUP08 - Universidade do Porto
- TAGS
- universidade
- porto
- ijup.up.pt
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
PHOTOCATALYTIC DEGRADATION OF CIPROFLOXACIN<br />
ANTIBIOTIC IN TiO2 AQUEOUS SUSPENSION<br />
F. G. Azeve<strong>do</strong>, M. Domingos, C. G. Silva and J. L. Faria<br />
Laboratório de Catálise e Materiais, Departamento de Engenharia Química, Faculdade de<br />
Engenharia, <strong>Universidade</strong> <strong>do</strong> <strong>Porto</strong>, Rua Dr. Roberto Frias 4200-465 <strong>Porto</strong>, Portugal<br />
The presence of pharmaceuticals and their metabolites in aquatic environments has raised<br />
increasing concern in recent years [1]. Antibiotics can hardly be degraded by biological<br />
processes, leading to the persistence of these compounds in the aquatic ecosystem posing a<br />
real threat in what concerns bio-accumulation. Hence, it is a priority to search for<br />
alternative chemical treatment methods, which can effectively eliminate the disposed<br />
amounts of this type of compounds. Ciprofloxacin (CIPRO) is an antibacterial drug<br />
belonging to the class of fluoroquinolones, widely used in the treatment of severe<br />
infections in humans. Among the possible treatments, TiO2-mediated photocatalysis has<br />
proven to be very effective in the complete oxidation of a great variety of organic<br />
pollutants [2]. The process is based in the oxidative potential of the hydroxyl radical<br />
(HO • ), which reacts rapidly and non-selectively with a wide range of organic compounds.<br />
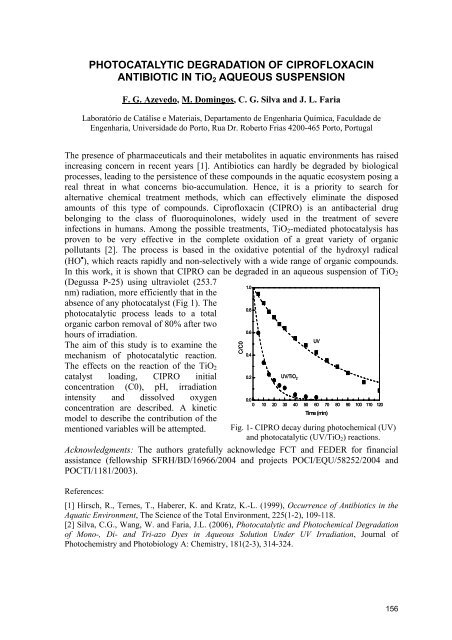
In this work, it is shown that CIPRO can be degraded in an aqueous suspension of TiO2<br />
(Degussa P-25) using ultraviolet (253.7<br />
nm) radiation, more efficiently that in the<br />
absence of any photocatalyst (Fig 1). The<br />
photocatalytic process leads to a total<br />
organic carbon removal of 80% after two<br />
hours of irradiation.<br />
The aim of this study is to examine the<br />
mechanism of photocatalytic reaction.<br />
The effects on the reaction of the TiO2<br />
catalyst loading, CIPRO initial<br />
concentration (C0), pH, irradiation<br />
intensity and dissolved oxygen<br />
concentration are described. A kinetic<br />
model to describe the contribution of the<br />
mentioned variables will be attempted.<br />
0.0<br />
0 10 20 30 40 50 60 70 80 90 100 110 120<br />
Acknowledgments: The authors gratefully acknowledge FCT and FEDER for financial<br />
assistance (fellowship SFRH/BD/16966/2004 and projects POCI/EQU/58252/2004 and<br />
POCTI/1181/2003).<br />
References:<br />
[1] Hirsch, R., Ternes, T., Haberer, K. and Kratz, K.-L. (1999), Occurrence of Antibiotics in the<br />
Aquatic Environment, The Science of the Total Environment, 225(1-2), 109-118.<br />
[2] Silva, C.G., Wang, W. and Faria, J.L. (2006), Photocatalytic and Photochemical Degradation<br />
of Mono-, Di- and Tri-azo Dyes in Aqueous Solution Under UV Irradiation, Journal of<br />
Photochemistry and Photobiology A: Chemistry, 181(2-3), 314-324.<br />
C/C0<br />
1.0<br />
0.8<br />
0.6<br />
0.4<br />
0.2<br />
UV/TiO 2<br />
UV<br />
Time (min)<br />
Fig. 1- CIPRO decay during photochemical (UV)<br />
and photocatalytic (UV/TiO2) reactions.<br />
156