IJUP08 - Universidade do Porto
IJUP08 - Universidade do Porto
IJUP08 - Universidade do Porto
- TAGS
- universidade
- porto
- ijup.up.pt
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Synthesis of xanthone derivates for in vitro and in vivo biological<br />
activity studies<br />
J. Siroka 1,2 , E. Sousa 2,3 and M. Pinto 2,3<br />
1 Department of Organic Chemistry, Faculty of Pharmacy in Hradec Králové, Charles University in<br />
Prague, Czech Republic.<br />
2 Department of Organic Chemistry, Faculty of Pharmacy, University of <strong>Porto</strong>, Portugal.<br />
3 Research Centre of Organic Chemistry, Phytochemistry and Pharmacology of the University of<br />
<strong>Porto</strong> (CEQOFFUP), Faculty of Pharmacy, University of <strong>Porto</strong>, Portugal<br />
Xanthone derivatives are heterocyclic compounds with the dibenzo-γ-pyrone as the main<br />
molecular moiety. They contain different types of substituents in different positions,<br />
leading to a large variety of pharmacological activities [1]. 3,4-Dihydroxyxanthone (1, Fig.<br />
1) was revealed as a hit compound in a study involving the investigation of the inhibitory<br />
effect of oxygenated xanthones on several human tumor cell lines [2].<br />
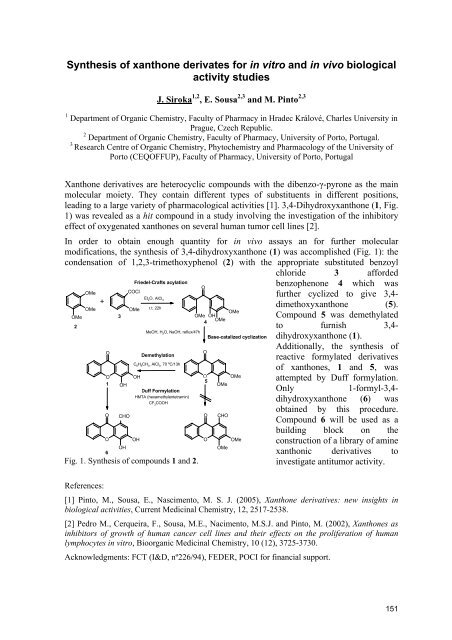
In order to obtain enough quantity for in vivo assays an for further molecular<br />
modifications, the synthesis of 3,4-dihydroxyxanthone (1) was accomplished (Fig. 1): the<br />
condensation of 1,2,3-trimethoxyphenol (2) with the appropriate substituted benzoyl<br />
chloride 3 afforded<br />
OMe<br />
OMe<br />
OMe<br />
2<br />
+<br />
O<br />
O<br />
1<br />
O<br />
O<br />
6<br />
3<br />
OH<br />
CHO<br />
HCO<br />
OH<br />
Friedel-Crafts acylation<br />
COCl<br />
Et2O, AlCl3 OMe<br />
OH<br />
Duff Formylation<br />
HMTA (hexamethylentetramin)<br />
CF3COOH OH<br />
r.t. 22h<br />
MeOH, H 2 O, NaOH, reflux/47h<br />
Demethylation<br />
C6H5CH3 , AlCl3 , 70 ºC/13h<br />
Fig. 1. Synthesis of compounds 1 and 2.<br />
O<br />
OMe<br />
OMe OH<br />
OMe<br />
4<br />
O<br />
O<br />
5<br />
O<br />
O<br />
Base-catalized cyclization<br />
OMe<br />
OMe<br />
CHO<br />
OMe<br />
OMe<br />
benzophenone 4 which was<br />
further cyclized to give 3,4dimethoxyxanthone<br />
(5).<br />
Compound 5 was demethylated<br />
to furnish 3,4dihydroxyxanthone<br />
(1).<br />
Additionally, the synthesis of<br />
reactive formylated derivatives<br />
of xanthones, 1 and 5, was<br />
attempted by Duff formylation.<br />
Only 1-formyl-3,4dihydroxyxanthone<br />
(6) was<br />
obtained by this procedure.<br />
Compound 6 will be used as a<br />
building block on the<br />
construction of a library of amine<br />
xanthonic derivatives to<br />
investigate antitumor activity.<br />
References:<br />
[1] Pinto, M., Sousa, E., Nascimento, M. S. J. (2005), Xanthone derivatives: new insights in<br />
biological activities, Current Medicinal Chemistry, 12, 2517-2538.<br />
[2] Pedro M., Cerqueira, F., Sousa, M.E., Nacimento, M.S.J. and Pinto, M. (2002), Xanthones as<br />
inhibitors of growth of human cancer cell lines and their effects on the proliferation of human<br />
lymphocytes in vitro, Bioorganic Medicinal Chemistry, 10 (12), 3725-3730.<br />
Acknowledgments: FCT (I&D, nº226/94), FEDER, POCI for financial support.<br />
151