IJUP08 - Universidade do Porto
IJUP08 - Universidade do Porto
IJUP08 - Universidade do Porto
- TAGS
- universidade
- porto
- ijup.up.pt
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Evaluation of primer coatings on steel subtracts by a low-cost,<br />
fast electrochemical technique<br />
V. Gonçalves 1, 2 , A. Mendes 1, 2 , J. Macha<strong>do</strong> 3 , F. Oliveira 3 , J. Nogueira 2, 3 , H.<br />
Aguilar Ribeiro 1<br />
1 LEPAE – Laboratory for Process, Environmental and Energy Engineering, Department of<br />
Chemical Engineering, Faculty of Engineering, University of <strong>Porto</strong>, Portugal.<br />
2 Rede de Competência em Polímeros, Faculty of Engineering, University of <strong>Porto</strong>, Portugal.<br />
3 CIN – Corporação Industrial <strong>do</strong> Norte, Maia, Portugal.<br />
Companies producing anticorrosive coatings for steel structures often need to test newly<br />
formulated products, to determine their actual behavior before large-scale production. In<br />
the coating industry, the most widely spread technique for assessing anticorrosive<br />
properties of a coating is still salt fog spray test. However, they give very subjective<br />
information and are very time and cost demanding. Modern electrochemical techniques,<br />
such as the electrochemical impedance spectroscopy (EIS), have made testing of the<br />
corrosion degradation behavior of painted metal systems a relatively simple matter, but the<br />
time to obtain an indication of the paint quality was still too long (1 week to 1 month) [1].<br />
In the present work we propose the use of an electrochemical test, known as “AC/DC/AC<br />
method” [1], to estimate the barrier properties of three different coatings, containing zinc<br />
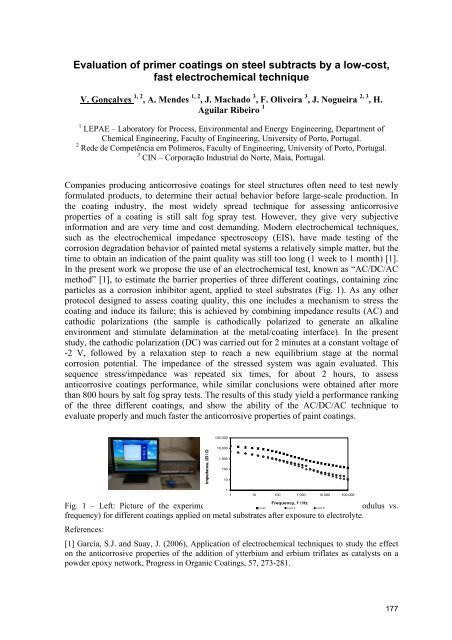
particles as a corrosion inhibitor agent, applied to steel substrates (Fig. 1). As any other<br />
protocol designed to assess coating quality, this one includes a mechanism to stress the<br />
coating and induce its failure; this is achieved by combining impedance results (AC) and<br />
cathodic polarizations (the sample is cathodically polarized to generate an alkaline<br />
environment and stimulate delamination at the metal/coating interface). In the present<br />
study, the cathodic polarization (DC) was carried out for 2 minutes at a constant voltage of<br />
-2 V, followed by a relaxation step to reach a new equilibrium stage at the normal<br />
corrosion potential. The impedance of the stressed system was again evaluated. This<br />
sequence stress/impedance was repeated six times, for about 2 hours, to assess<br />
anticorrosive coatings performance, while similar conclusions were obtained after more<br />
than 800 hours by salt fog spray tests. The results of this study yield a performance ranking<br />
of the three different coatings, and show the ability of the AC/DC/AC technique to<br />
evaluate properly and much faster the anticorrosive properties of paint coatings.<br />
Impedance, IZI / Ω<br />
100,000<br />
10,000<br />
1,000<br />
100<br />
10<br />
1<br />
1 10 100 1,000 10,000 100,000<br />
Frequency, f / Hz<br />
Fig. 1 – Left: Picture of the experimental set-up; Right: Level I Bode Level IIplot (impedance Level III<br />
modulus vs.<br />
frequency) for different coatings applied on metal substrates after exposure to electrolyte.<br />
References:<br />
[1] García, S.J. and Suay, J. (2006), Application of electrochemical techniques to study the effect<br />
on the anticorrosive properties of the addition of ytterbium and erbium triflates as catalysts on a<br />
powder epoxy network, Progress in Organic Coatings, 57, 273-281.<br />
177