My PhD dissertation - Institut Fresnel
My PhD dissertation - Institut Fresnel
My PhD dissertation - Institut Fresnel
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
99<br />
To illustrate the experimental method for the quantification of the phosphine oxides<br />
resulting from alkaline decomposition of phosphonium halides, a detailed protocole is given<br />
for tributylneopentylphosphonium iodide degradation:<br />
Tributylneopentylphosphonium iodide (0.40 g, 1.0 mmol) was suspended in butanol (5.0 mL) and<br />
a 6.0 M aqueous potassium hydroxide solution (1.0 mL) was added. Triisopropylphosphine oxide<br />
(0.13 g, 0.76 mmol) was immediately added as internal standard. The reaction mixture was then<br />
heated at 110 °C in a thermostated bath until no trace of starting phosphonium remained. The gases<br />
formed during the decomposition were bubbled into a solution of bromine (0.2 mL, 0.5 g, 3 mmol) in<br />
chloroform (5.0 mL). After the gas evolution ceased, the bromine solution was treated with a sodium<br />
sulfite solution, phases were decantated. The organic layer was dried with sodium sulfate and the<br />
presence of brominated adduct was immediately checked by gas chromatography (30 m, DB-1,<br />
70 °C). Cyclohexane (20 mL) was added to the reaction slurry and the solvents were removed under<br />
reduced pressure. The residue was dissolved in diethyl ether (15 mL), dried with sodium sulfate and<br />
evaporated to dryness. 31 P NMR experiment was directly run on the crude mixture without any further<br />
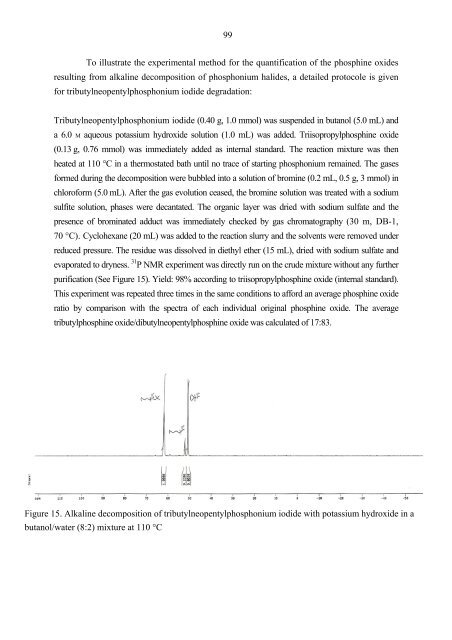
purification (See Figure 15). Yield: 98% according to triisopropylphosphine oxide (internal standard).<br />
This experiment was repeated three times in the same conditions to afford an average phosphine oxide<br />
ratio by comparison with the spectra of each individual original phosphine oxide. The average<br />
tributylphosphine oxide/dibutylneopentylphosphine oxide was calculated of 17:83.<br />
Figure 15. Alkaline decomposition of tributylneopentylphosphonium iodide with potassium hydroxide in a<br />
butanol/water (8:2) mixture at 110 °C