My PhD dissertation - Institut Fresnel
My PhD dissertation - Institut Fresnel
My PhD dissertation - Institut Fresnel
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
33<br />
were comparable to those obtained with a more classical butanol/water (8 : 2) mixture at<br />
110 °C, and removal of the alcohol during work up was more convenient, this alcoholic<br />
reaction media was chosen instead.<br />
This study was not meant to be exhaustive but tried to select pertinent starting<br />
substrates which allowed the comparison of primary, secondary and tertiary alkyl anion<br />
stabilities in a systematic manner. Thus different primary alkyl carbanions were first<br />
compared with each other, by studying the alkaline decomposition of three<br />
tetraalkylphosphonium iodides bearing only primary alkyl groups. Decomposition of<br />
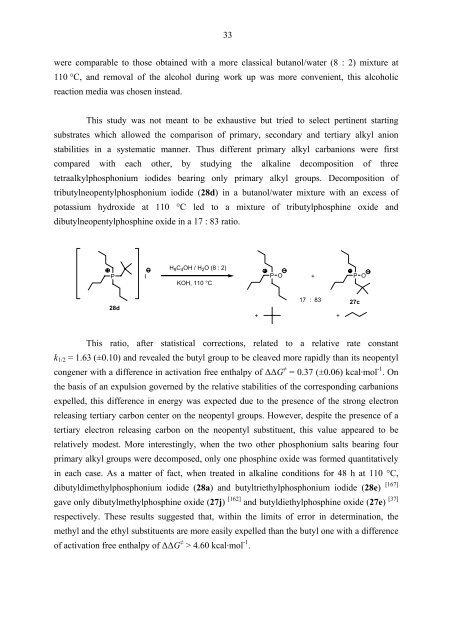
tributylneopentylphosphonium iodide (28d) in a butanol/water mixture with an excess of<br />
potassium hydroxide at 110 °C led to a mixture of tributylphosphine oxide and<br />
dibutylneopentylphosphine oxide in a 17 : 83 ratio.<br />
H9C4OH / H2O (8 : 2)<br />
P I P O +<br />
P O<br />
KOH, 110 °C<br />
28d<br />
17 : 83<br />
+ +<br />
This ratio, after statistical corrections, related to a relative rate constant<br />
k1/2 = 1.63 (±0.10) and revealed the butyl group to be cleaved more rapidly than its neopentyl<br />
congener with a difference in activation free enthalpy of ∆∆G ≠ = 0.37 (±0.06) kcal·mol -1 . On<br />
the basis of an expulsion governed by the relative stabilities of the corresponding carbanions<br />
expelled, this difference in energy was expected due to the presence of the strong electron<br />
releasing tertiary carbon center on the neopentyl groups. However, despite the presence of a<br />
tertiary electron releasing carbon on the neopentyl substituent, this value appeared to be<br />
relatively modest. More interestingly, when the two other phosphonium salts bearing four<br />
primary alkyl groups were decomposed, only one phosphine oxide was formed quantitatively<br />
in each case. As a matter of fact, when treated in alkaline conditions for 48 h at 110 °C,<br />
dibutyldimethylphosphonium iodide (28a) and butyltriethylphosphonium iodide (28e)<br />
gave only dibutylmethylphosphine oxide (27j) [162] and butyldiethylphosphine oxide (27e) [37]<br />
respectively. These results suggested that, within the limits of error in determination, the<br />
methyl and the ethyl substituents are more easily expelled than the butyl one with a difference<br />
of activation free enthalpy of ∆∆G ≠ > 4.60 kcal·mol -1 .<br />
27c<br />
[ ] 167