My PhD dissertation - Institut Fresnel
My PhD dissertation - Institut Fresnel
My PhD dissertation - Institut Fresnel
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
1 Introduction<br />
1<br />
The present thesis work aims at linking organophosphorus chemistry and modern<br />
organometallic techniques. Thus, phosphorus compounds will not only serve as targets of<br />
synthesis obtained through organometallic methods, but also as probes for gaining better<br />
insight into organometallic reactivity. One main objective is the preparation of new phosphine<br />
ligands for transition-metal-catalyzed reactions. In the course of this work, thermodynamical<br />
investigations will also be performed in order to assess the rotational stability of some<br />
atropisomeric biphenyl substrates. On the other hand, tetraalkylphosphonium salt will serve as<br />
a substrate for alkaline decomposition, allowing us to compare relative basicities of various<br />
alkyl anions.<br />
1.1 Atropisomerism and Axially Chiral Bisphosphine<br />
Ligands<br />
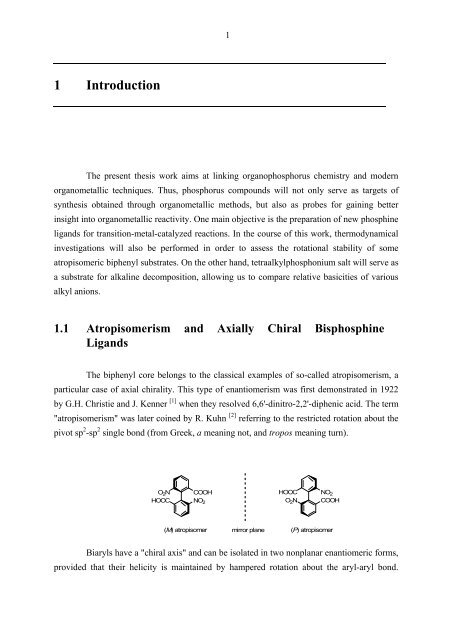
The biphenyl core belongs to the classical examples of so-called atropisomerism, a<br />
particular case of axial chirality. This type of enantiomerism was first demonstrated in 1922<br />
by G.H. Christie and J. Kenner<br />
"atropisomerism" was later coined by R. Kuhn<br />
[ ] 1 when they resolved 6,6'-dinitro-2,2'-diphenic acid. The term<br />
[ ] 2 referring to the restricted rotation about the<br />
pivot sp 2 -sp 2 single bond (from Greek, a meaning not, and tropos meaning turn).<br />
O2N HOOC<br />
COOH<br />
NO2 HOOC NO2 O2N COOH<br />
(M) atropisomer mirror plane (P) atropisomer<br />
Biaryls have a "chiral axis" and can be isolated in two nonplanar enantiomeric forms,<br />
provided that their helicity is maintained by hampered rotation about the aryl-aryl bond.