My PhD dissertation - Institut Fresnel
My PhD dissertation - Institut Fresnel
My PhD dissertation - Institut Fresnel
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
61<br />
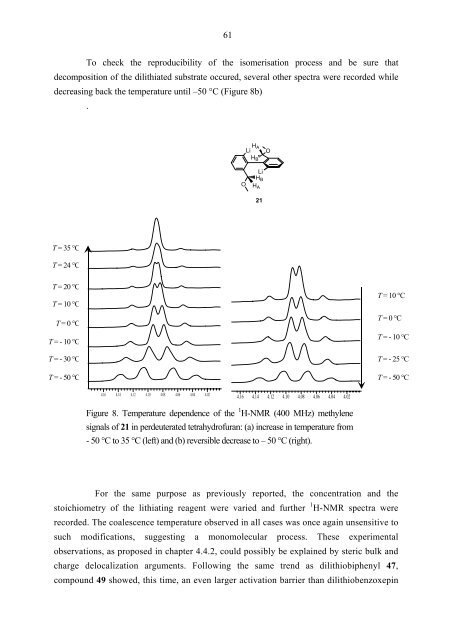
To check the reproducibility of the isomerisation process and be sure that<br />
decomposition of the dilithiated substrate occured, several other spectra were recorded while<br />
decreasing back the temperature until –50 °C (Figure 8b)<br />
T = 35 °C<br />
T = 24 °C<br />
T = 20 °C<br />
T = 10 °C<br />
T = 0 °C<br />
T = - 10 °C<br />
.<br />
O<br />
HA Li<br />
HB Li<br />
HB HA 21<br />
O<br />
T = 10 °C<br />
T = 0 °C<br />
T = - 10 °C<br />
T = - 30 °C T = - 25 °C<br />
T = - 50 °C T = - 50 °C<br />
4.16 4.14 4.12 4.10 4.08 4.06 4.04 4.02<br />
4.16 4.14 4.12 4.10 4.08 4.06 4.04 4.02<br />
Figure 8. Temperature dependence of the 1 H-NMR (400 MHz) methylene<br />
signals of 21 in perdeuterated tetrahydrofuran: (a) increase in temperature from<br />
- 50 °C to 35 °C (left) and (b) reversible decrease to – 50 °C (right).<br />
For the same purpose as previously reported, the concentration and the<br />
stoichiometry of the lithiating reagent were varied and further 1 H-NMR spectra were<br />
recorded. The coalescence temperature observed in all cases was once again unsensitive to<br />
such modifications, suggesting a monomolecular process. These experimental<br />
observations, as proposed in chapter 4.4.2, could possibly be explained by steric bulk and<br />
charge delocalization arguments. Following the same trend as dilithiobiphenyl 47,<br />
compound 49 showed, this time, an even larger activation barrier than dilithiobenzoxepin