My PhD dissertation - Institut Fresnel
My PhD dissertation - Institut Fresnel
My PhD dissertation - Institut Fresnel
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
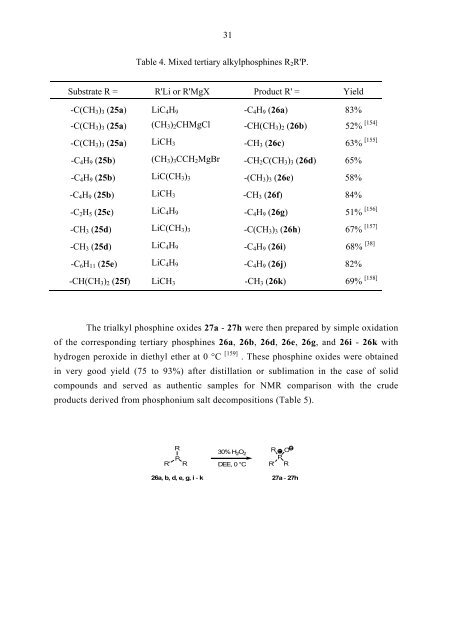
31<br />
Table 4. Mixed tertiary alkylphosphines R2R'P.<br />
Substrate R = R'Li or R'MgX Product R' = Yield<br />
-C(CH3)3 (25a) LiC4H9 -C4H9 (26a) 83%<br />
-C(CH3)3 (25a) (CH3)2CHMgCl -CH(CH3)2 (26b) 52%<br />
-C(CH3)3 (25a) LiCH3 -CH3 (26c) 63%<br />
-C4H9 (25b) (CH3)3CCH2MgBr -CH2C(CH3)3 (26d) 65%<br />
-C4H9 (25b) LiC(CH3)3 -(CH3)3 (26e) 58%<br />
-C4H9 (25b) LiCH3 -CH3 (26f) 84%<br />
-C2H5 (25c) LiC4H9 -C4H9 (26g) 51%<br />
-CH3 (25d) LiC(CH3)3 -C(CH3)3 (26h) 67%<br />
-CH3 (25d) LiC4H9 -C4H9 (26i) 68% [38]<br />
-C6H11 (25e) LiC4H9 -C4H9 (26j) 82%<br />
-CH(CH3)2 (25f) LiCH3 -CH3 (26k) 69%<br />
The trialkyl phosphine oxides 27a - 27h were then prepared by simple oxidation<br />
of the corresponding tertiary phosphines 26a, 26b, 26d, 26e, 26g, and 26i - 26k with<br />
hydrogen peroxide in diethyl ether at 0 °C<br />
[ ] 154<br />
[ ] 155<br />
[ ] 156<br />
[ ] 157<br />
[ ] 158<br />
[ ] 159 . These phosphine oxides were obtained<br />
in very good yield (75 to 93%) after distillation or sublimation in the case of solid<br />
compounds and served as authentic samples for NMR comparison with the crude<br />
products derived from phosphonium salt decompositions (Table 5).<br />
R'<br />
R<br />
P R<br />
26a, b, d, e, g, i - k<br />
30% H2O 2<br />
DEE, 0 °C<br />
R O<br />
P<br />
R' R<br />
27a - 27h