My PhD dissertation - Institut Fresnel
My PhD dissertation - Institut Fresnel
My PhD dissertation - Institut Fresnel
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
20<br />
organic molecules have been determined [113 - 116] , those of many of the simplest, namely<br />
the linear and cyclic alkanes, remain to be established. This specific lack of information<br />
accounts for the very weak acidity of such compounds which hardly generate the<br />
corresponding carbanion in the gas phase<br />
[ ] 117 and thus do not afford any thermodynamic<br />
°<br />
data evaluation. The exact acidity of methane ∆H CH ) [i.e. proton affinity, PA(CH4)]<br />
acid ( 4<br />
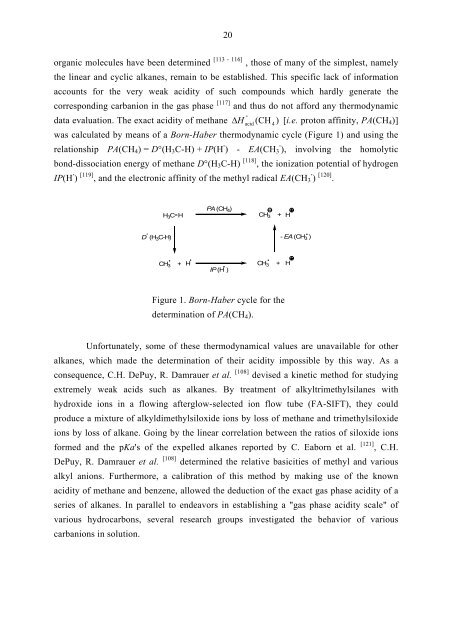
was calculated by means of a Born-Haber thermodynamic cycle (Figure 1) and using the<br />
relationship PA(CH4) = D°(H3C-H) + IP(H · ) - EA(CH3 · ), involving the homolytic<br />
bond-dissociation energy of methane D°(H3C-H) [118] , the ionization potential of hydrogen<br />
IP(H · ) [119] , and the electronic affinity of the methyl radical EA(CH3 · ) [120] .<br />
D ° (H 3C-H)<br />
PA (CH 4)<br />
H3CH CH3 + H<br />
CH 3<br />
+ H<br />
CH3 +<br />
IP (H )<br />
Figure 1. Born-Haber cycle for the<br />
determination of PA(CH4).<br />
- EA (CH 3 )<br />
Unfortunately, some of these thermodynamical values are unavailable for other<br />
alkanes, which made the determination of their acidity impossible by this way. As a<br />
consequence, C.H. DePuy, R. Damrauer et al. [108] devised a kinetic method for studying<br />
extremely weak acids such as alkanes. By treatment of alkyltrimethylsilanes with<br />
hydroxide ions in a flowing afterglow-selected ion flow tube (FA-SIFT), they could<br />
produce a mixture of alkyldimethylsiloxide ions by loss of methane and trimethylsiloxide<br />
ions by loss of alkane. Going by the linear correlation between the ratios of siloxide ions<br />
formed and the pKa's of the expelled alkanes reported by C. Eaborn et al. [121] , C.H.<br />
DePuy, R. Damrauer et al. [108] determined the relative basicities of methyl and various<br />
alkyl anions. Furthermore, a calibration of this method by making use of the known<br />
acidity of methane and benzene, allowed the deduction of the exact gas phase acidity of a<br />
series of alkanes. In parallel to endeavors in establishing a "gas phase acidity scale" of<br />
various hydrocarbons, several research groups investigated the behavior of various<br />
carbanions in solution.<br />
H