My PhD dissertation - Institut Fresnel
My PhD dissertation - Institut Fresnel
My PhD dissertation - Institut Fresnel
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
47<br />
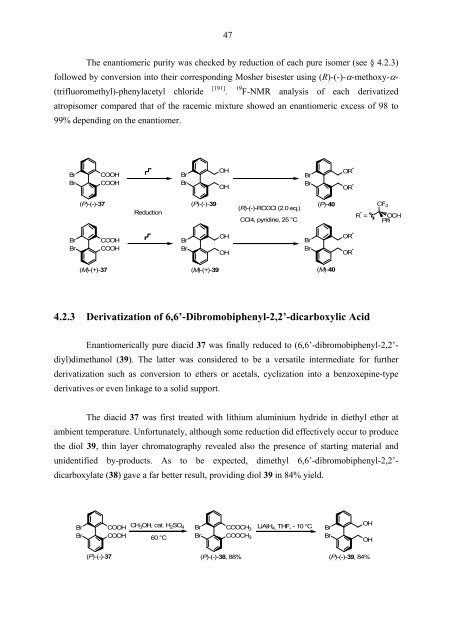
The enantiomeric purity was checked by reduction of each pure isomer (see § 4.2.3)<br />
followed by conversion into their corresponding Mosher bisester using (R)-(-)-α-methoxy-α-<br />
(trifluoromethyl)-phenylacetyl chloride<br />
[ 191 ]<br />
.<br />
19 F-NMR analysis of each derivatized<br />
atropisomer compared that of the racemic mixture showed an enantiomeric excess of 98 to<br />
99% depending on the enantiomer.<br />
Br<br />
Br<br />
(P)-(-)-37<br />
COOH<br />
COOH<br />
Br COOH<br />
Br COOH<br />
(M)-(+)-37<br />
Reduction<br />
Br<br />
Br<br />
Br<br />
Br<br />
(P)-(-)-39<br />
(M)-(+)-39<br />
OH<br />
OH<br />
OH<br />
OH<br />
(R)-(-)-RCOCl (2.0 eq.)<br />
CCl4, pyridine, 25 °C<br />
Br<br />
Br<br />
Br<br />
Br<br />
(P)-40<br />
(M)-40<br />
4.2.3 Derivatization of 6,6’-Dibromobiphenyl-2,2’-dicarboxylic Acid<br />
OR *<br />
OR *<br />
OR *<br />
OR *<br />
R * =<br />
CF 3<br />
OCH<br />
Ph3<br />
Enantiomerically pure diacid 37 was finally reduced to (6,6’-dibromobiphenyl-2,2’-<br />
diyl)dimethanol (39). The latter was considered to be a versatile intermediate for further<br />
derivatization such as conversion to ethers or acetals, cyclization into a benzoxepine-type<br />
derivatives or even linkage to a solid support.<br />
The diacid 37 was first treated with lithium aluminium hydride in diethyl ether at<br />
ambient temperature. Unfortunately, although some reduction did effectively occur to produce<br />
the diol 39, thin layer chromatography revealed also the presence of starting material and<br />
unidentified by-products. As to be expected, dimethyl 6,6’-dibromobiphenyl-2,2’-<br />
dicarboxylate (38) gave a far better result, providing diol 39 in 84% yield.<br />
Br<br />
Br<br />
(P)-(-)-37<br />
COOH<br />
COOH<br />
CH 3OH, cat. H 2SO 4<br />
60 °C<br />
Br<br />
Br<br />
COOCH 3<br />
COOCH 3<br />
(P)-(-)-38, 88%<br />
LiAlH 4, THF, - 10 °C<br />
Br<br />
Br<br />
OH<br />
OH<br />
(P)-(-)-39, 84%