My PhD dissertation - Institut Fresnel
My PhD dissertation - Institut Fresnel
My PhD dissertation - Institut Fresnel
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
50<br />
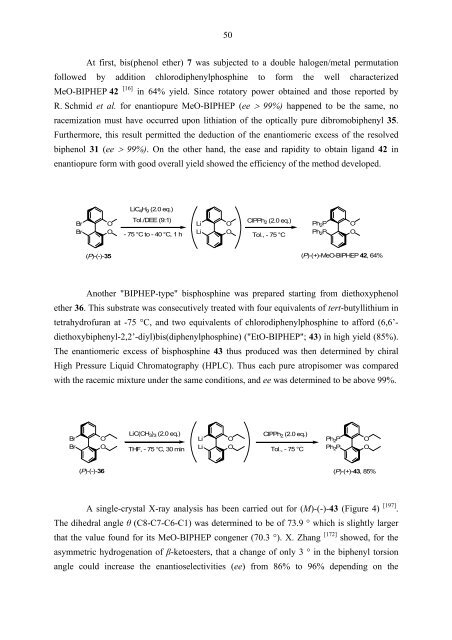
At first, bis(phenol ether) 7 was subjected to a double halogen/metal permutation<br />
followed by addition chlorodiphenylphosphine to form the well characterized<br />
MeO-BIPHEP 42 [16] in 64% yield. Since rotatory power obtained and those reported by<br />
R. Schmid et al. for enantiopure MeO-BIPHEP (ee > 99%) happened to be the same, no<br />
racemization must have occurred upon lithiation of the optically pure dibromobiphenyl 35.<br />
Furthermore, this result permitted the deduction of the enantiomeric excess of the resolved<br />
biphenol 31 (ee > 99%). On the other hand, the ease and rapidity to obtain ligand 42 in<br />
enantiopure form with good overall yield showed the efficiency of the method developed.<br />
Br<br />
Br<br />
O<br />
O<br />
(P)-(-)-35<br />
LiC 4H 9 (2.0 eq.)<br />
Tol./DEE (9:1)<br />
- 75 °C to - 40 °C, 1 h<br />
Li<br />
Li<br />
O<br />
O<br />
ClPPh 2 (2.0 eq.)<br />
Tol., - 75 °C<br />
Ph2P Ph2P O<br />
O<br />
(P)-(+)-MeO-BIPHEP 42, 64%<br />
Another "BIPHEP-type" bisphosphine was prepared starting from diethoxyphenol<br />
ether 36. This substrate was consecutively treated with four equivalents of tert-butyllithium in<br />
tetrahydrofuran at -75 °C, and two equivalents of chlorodiphenylphosphine to afford (6,6’-<br />
diethoxybiphenyl-2,2’-diyl)bis(diphenylphosphine) ("EtO-BIPHEP"; 43) in high yield (85%).<br />
The enantiomeric excess of bisphosphine 43 thus produced was then determined by chiral<br />
High Pressure Liquid Chromatography (HPLC). Thus each pure atropisomer was compared<br />
with the racemic mixture under the same conditions, and ee was determined to be above 99%.<br />
Br<br />
Br<br />
O<br />
O<br />
(P)-(-)-36<br />
LiC(CH 3) 3 (2.0 eq.)<br />
THF, - 75 °C, 30 min<br />
Li<br />
Li<br />
O<br />
O<br />
ClPPh 2 (2.0 eq.)<br />
Tol., - 75 °C<br />
Ph2P Ph2P O<br />
O<br />
(P)-(+)-43, 85%<br />
A single-crystal X-ray analysis has been carried out for (M)-(-)-43 (Figure 4)<br />
The dihedral angle θ (C8-C7-C6-C1) was determined to be of 73.9 ° which is slightly larger<br />
that the value found for its MeO-BIPHEP congener (70.3 °). X. Zhang [172] showed, for the<br />
asymmetric hydrogenation of β-ketoesters, that a change of only 3 ° in the biphenyl torsion<br />
angle could increase the enantioselectivities (ee) from 86% to 96% depending on the<br />
[ ] 197 .