My PhD dissertation - Institut Fresnel
My PhD dissertation - Institut Fresnel
My PhD dissertation - Institut Fresnel
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
51<br />
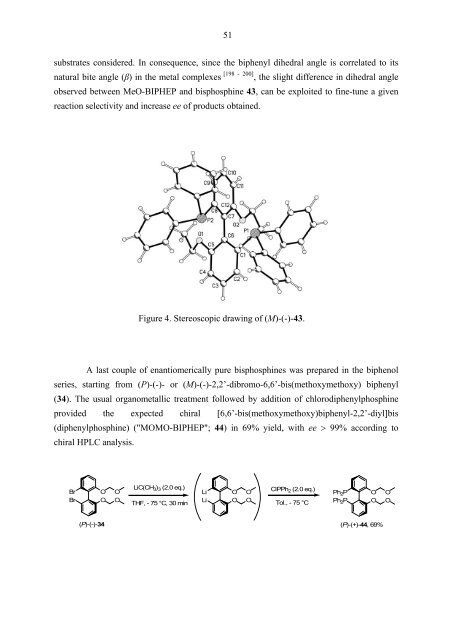
substrates considered. In consequence, since the biphenyl dihedral angle is correlated to its<br />
natural bite angle (β) in the metal complexes [198 - 200] , the slight difference in dihedral angle<br />
observed between MeO-BIPHEP and bisphosphine 43, can be exploited to fine-tune a given<br />
reaction selectivity and increase ee of products obtained.<br />
Figure 4. Stereoscopic drawing of (M)-(-)-43.<br />
A last couple of enantiomerically pure bisphosphines was prepared in the biphenol<br />
series, starting from (P)-(-)- or (M)-(-)-2,2’-dibromo-6,6’-bis(methoxymethoxy) biphenyl<br />
(34). The usual organometallic treatment followed by addition of chlorodiphenylphosphine<br />
provided the expected chiral [6,6’-bis(methoxymethoxy)biphenyl-2,2’-diyl]bis<br />
(diphenylphosphine) ("MOMO-BIPHEP"; 44) in 69% yield, with ee > 99% according to<br />
chiral HPLC analysis.<br />
Br<br />
Br<br />
O<br />
O<br />
(P)-(-)-34<br />
O<br />
O<br />
LiC(CH 3) 3 (2.0 eq.)<br />
THF, - 75 °C, 30 min<br />
Li<br />
Li<br />
O<br />
O<br />
O<br />
O<br />
ClPPh 2 (2.0 eq.)<br />
Tol., - 75 °C<br />
Ph2P Ph2P O<br />
O<br />
(P)-(+)-44, 69%<br />
O<br />
O