My PhD dissertation - Institut Fresnel
My PhD dissertation - Institut Fresnel
My PhD dissertation - Institut Fresnel
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
17<br />
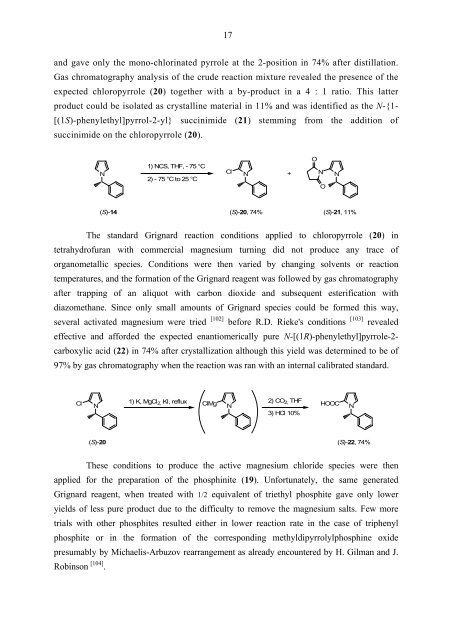
and gave only the mono-chlorinated pyrrole at the 2-position in 74% after distillation.<br />
Gas chromatography analysis of the crude reaction mixture revealed the presence of the<br />
expected chloropyrrole (20) together with a by-product in a 4 : 1 ratio. This latter<br />
product could be isolated as crystalline material in 11% and was identified as the N-{1-<br />
[(1S)-phenylethyl]pyrrol-2-yl} succinimide (21) stemming from the addition of<br />
succinimide on the chloropyrrole (20).<br />
N<br />
(S)-14<br />
1) NCS, THF, - 75 °C<br />
2) - 75 °C to 25 °C<br />
Cl<br />
N + N N<br />
(S)-20, 74% (S)-21, 11%<br />
The standard Grignard reaction conditions applied to chloropyrrole (20) in<br />
tetrahydrofuran with commercial magnesium turning did not produce any trace of<br />
organometallic species. Conditions were then varied by changing solvents or reaction<br />
temperatures, and the formation of the Grignard reagent was followed by gas chromatography<br />
after trapping of an aliquot with carbon dioxide and subsequent esterification with<br />
diazomethane. Since only small amounts of Grignard species could be formed this way,<br />
several activated magnesium were tried<br />
[ ] 102 before R.D. Rieke's conditions<br />
O<br />
O<br />
[ ] 103 revealed<br />
effective and afforded the expected enantiomerically pure N-[(1R)-phenylethyl]pyrrole-2-<br />
carboxylic acid (22) in 74% after crystallization although this yield was determined to be of<br />
97% by gas chromatography when the reaction was ran with an internal calibrated standard.<br />
Cl<br />
N<br />
(S)-20<br />
1) K, MgCl 2, KI, reflux<br />
ClMg<br />
N<br />
2) CO 2, THF<br />
3) HCl 10%<br />
HOOC<br />
N<br />
(S)-22, 74%<br />
These conditions to produce the active magnesium chloride species were then<br />
applied for the preparation of the phosphinite (19). Unfortunately, the same generated<br />
Grignard reagent, when treated with 1/2 equivalent of triethyl phosphite gave only lower<br />
yields of less pure product due to the difficulty to remove the magnesium salts. Few more<br />
trials with other phosphites resulted either in lower reaction rate in the case of triphenyl<br />
phosphite or in the formation of the corresponding methyldipyrrolylphosphine oxide<br />
presumably by Michaelis-Arbuzov rearrangement as already encountered by H. Gilman and J.<br />
Robinson<br />
[ ] 104 .