My PhD dissertation - Institut Fresnel
My PhD dissertation - Institut Fresnel
My PhD dissertation - Institut Fresnel
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
41<br />
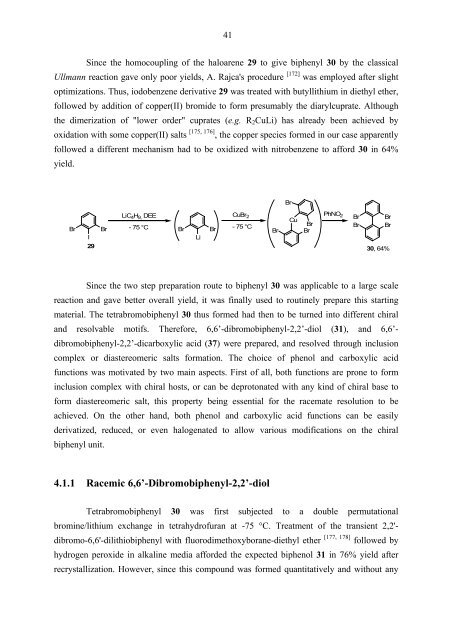
Since the homocoupling of the haloarene 29 to give biphenyl 30 by the classical<br />
Ullmann reaction gave only poor yields, A. Rajca's procedure [172] was employed after slight<br />
optimizations. Thus, iodobenzene derivative 29 was treated with butyllithium in diethyl ether,<br />
followed by addition of copper(II) bromide to form presumably the diarylcuprate. Although<br />
the dimerization of "lower order" cuprates (e.g. R2CuLi) has already been achieved by<br />
oxidation with some copper(II) salts<br />
[ , ] 175 176 , the copper species formed in our case apparently<br />
followed a different mechanism had to be oxidized with nitrobenzene to afford 30 in 64%<br />
yield.<br />
Br<br />
I<br />
29<br />
Br<br />
LiC 4H9, DEE<br />
- 75 °C Br Br<br />
CuBr 2<br />
- 75 °C<br />
Br<br />
Cu<br />
Br<br />
Br<br />
PhNO 2<br />
Li<br />
Br<br />
Br Br<br />
Br Br<br />
30, 64%<br />
Since the two step preparation route to biphenyl 30 was applicable to a large scale<br />
reaction and gave better overall yield, it was finally used to routinely prepare this starting<br />
material. The tetrabromobiphenyl 30 thus formed had then to be turned into different chiral<br />
and resolvable motifs. Therefore, 6,6’-dibromobiphenyl-2,2’-diol (31), and 6,6’-<br />
dibromobiphenyl-2,2’-dicarboxylic acid (37) were prepared, and resolved through inclusion<br />
complex or diastereomeric salts formation. The choice of phenol and carboxylic acid<br />
functions was motivated by two main aspects. First of all, both functions are prone to form<br />
inclusion complex with chiral hosts, or can be deprotonated with any kind of chiral base to<br />
form diastereomeric salt, this property being essential for the racemate resolution to be<br />
achieved. On the other hand, both phenol and carboxylic acid functions can be easily<br />
derivatized, reduced, or even halogenated to allow various modifications on the chiral<br />
biphenyl unit.<br />
4.1.1 Racemic 6,6’-Dibromobiphenyl-2,2’-diol<br />
Tetrabromobiphenyl 30 was first subjected to a double permutational<br />
bromine/lithium exchange in tetrahydrofuran at -75 °C. Treatment of the transient 2,2'-<br />
dibromo-6,6'-dilithiobiphenyl with fluorodimethoxyborane-diethyl ether<br />
[ , ] 177 178 followed by<br />
hydrogen peroxide in alkaline media afforded the expected biphenol 31 in 76% yield after<br />
recrystallization. However, since this compound was formed quantitatively and without any