My PhD dissertation - Institut Fresnel
My PhD dissertation - Institut Fresnel
My PhD dissertation - Institut Fresnel
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
5<br />
However, this mechanism suffers from inconsistencies since some cyclic substrates<br />
were found to show partial racemization or complete retention of stereochemistry at<br />
phosphorus<br />
[ , ] 41 42 . These results could be explained by ring strain. Phosphetanium (four-<br />
membered ring) and phospholanium (five-membered ring) salts preferentially adopt, in the<br />
trigonal bipyramidal intermediates, an equatorial-apical relation (90 °) due to angle constraint.<br />
Since apical positions are assumed to be favored for the departing group and the hydroxide<br />
[ 43 ]<br />
[ 44 ]<br />
, a Berry pseudorotation must mediate the move of the more stabilized anion to the<br />
apical position. The latter pseudorotation thus accounted for the partial retention of<br />
configuration at phosphorus center. In summary, even if applicable to non cyclic cases, this<br />
general explanation of the observed stereochemistry for the alkaline decomposition of<br />
phosphonium salts does not illustrate the whole scheme of this process.<br />
1.3 Objectives of this Work<br />
Organometallic techniques, particularly the halogen/metal permutation<br />
proved to be the method of choice in many areas of organic synthesis<br />
[ , ] 45 46 , have<br />
[ ] 47 . This is especially<br />
remarkable when it comes to organophosphorus chemistry and the preparation of tertiary<br />
phosphine ligands. Thus, phosphorus will constitute a leitmotiv in the present thesis work, and<br />
several aspects of its chemistry and applications will be taken into account.<br />
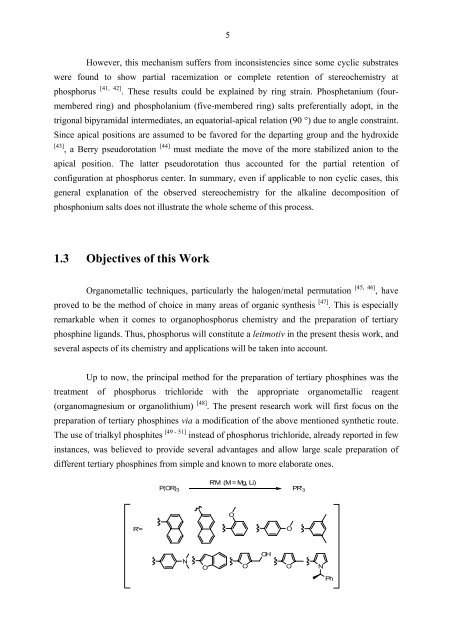
Up to now, the principal method for the preparation of tertiary phosphines was the<br />
treatment of phosphorus trichloride with the appropriate organometallic reagent<br />
(organomagnesium or organolithium)<br />
[ ] 48 . The present research work will first focus on the<br />
preparation of tertiary phosphines via a modification of the above mentioned synthetic route.<br />
The use of trialkyl phosphites<br />
[ - ] 49 51 instead of phosphorus trichloride, already reported in few<br />
instances, was believed to provide several advantages and allow large scale preparation of<br />
different tertiary phosphines from simple and known to more elaborate ones.<br />
R'=<br />
P(OR) 3<br />
N<br />
O<br />
R'M (M = Mg, Li)<br />
O<br />
O<br />
OH<br />
O<br />
O<br />
PR' 3<br />
N<br />
Ph